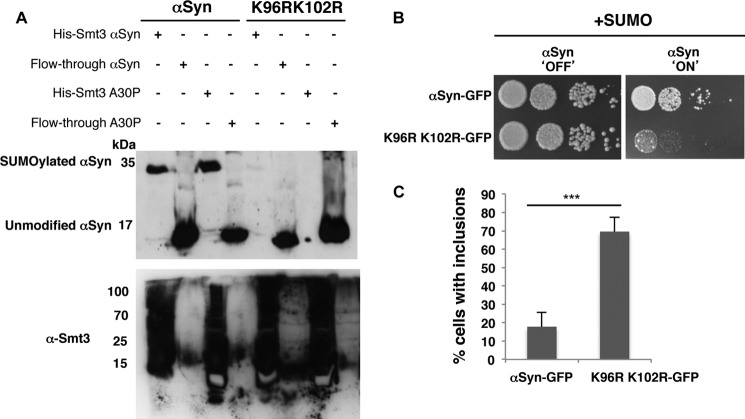
FIGURE 2.
Lysine 96 and 102 are conserved as major sumoylation sites of αSyn in eukaryotes. A, lysine to arginine substitutions at positions 96 and 102 resulted in decreased αSyn sumoylation. αSyn and A30PSyn and the corresponding αSyn amino acid variants K96R/K102R were transformed into ulp1ts yeast cells expressing the yeast SUMO protein His6-Smt3. His6-tagged SUMO conjugates were pulled down by Ni2+-NTA. αSyn was detected by Western hybridization using αSyn antibody (upper panel). Western hybridization of the same blot with Smt3 antibody (α-Smt3) verified the Ni2+-NTA pulldown (lower panel). B, spotting assay of W303 yeast cells, carrying two copies of GAL1-driven αSyn-GFP and K96R/K102R-GFP. Yeast cells were spotted in 10-fold dilutions on selection plates containing glucose (αSyn 'OFF') or galactose (αSyn 'ON'). C, quantification of the percentage of cells displaying αSyn inclusions in W303 yeast background. Significance of differences was calculated with t test (***, p < 0.001, n = 3).