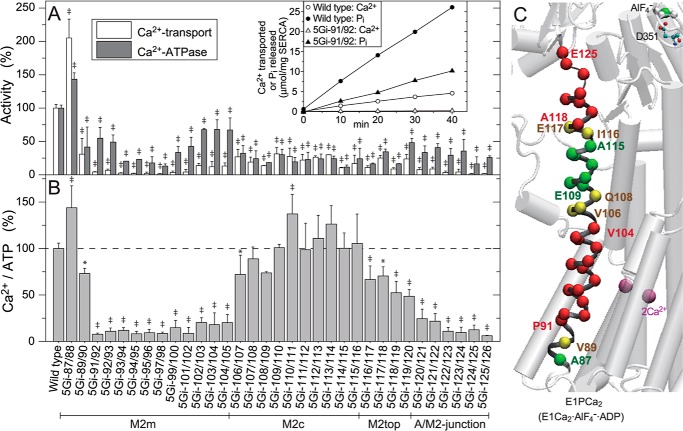
FIGURE 3.
Ca2+-ATPase and oxalate-dependent Ca2+ transport activities. A, the specific activities of the expressed SERCA1a 5Gi mutants were determined and shown as values relative to the respective wild-type activities (ATP hydrolysis, 0.594 ± 0.028 μmol of Pi/min/mg of SERCA1a protein (n = 5); oxalate-dependent Ca2+ transport, 0.116 ± 0.006 μmol Ca2+/min/mg SERCA1a protein (n = 5); very similar to the values obtained by our group and other groups under optimum conditions with the microsomes prepared from the COS cells (e.g. see Refs. 29, 47–49)). Typical time courses of Pi liberation and Ca2+ accumulation in the wild type and mutant 5Gi-91/92 are shown in the inset. B, the coupling ratio (i.e. Ca2+ transport activity per Ca2+-ATPase activity (Ca2+/ATP)), is shown as a percentage of the wild-type ratio. In A and B, statistical significance compared with the respective wild-type value is shown; *, p < 0.05; ‡, p < 0.01. C, the mutational effects of residues in B are visualized with α-carbon coloring on M2; green, Ca2+/ATP higher than 80% of the wild type (coupled transport); yellow, 80 to 60% (slightly uncoupled); red, less than 60% (severely uncoupled). Error bars, S.D.