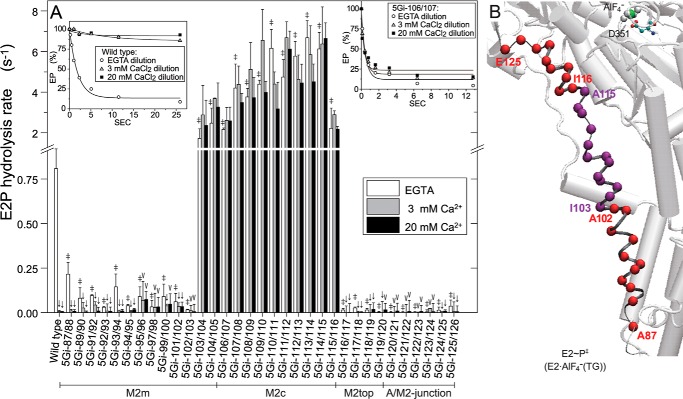
FIGURE 7.
E2P hydrolysis and luminal Ca2+-induced inhibition. A, microsomes expressing wild type or mutant were phosphorylated with 32Pi at 25 °C for 10 min in 5 μl of a mixture containing 1–5 μg of microsomal protein, 0.1 mm 32Pi, 1 μm A23187, 0.2 mm EGTA, 7 mm MgCl2, 50 mm MOPS/Tris (pH 7.0), and 35% (v/v) Me2SO. The mixture was then cooled and diluted at 0 °C by the addition of 95 μl of a mixture containing 2.1 mm non-radioactive Pi, 105 mm KCl, 7 mm MgCl2, 50 mm MOPS/Tris (pH 7.0), and 3 mm EGTA (open bar) or 3.2 mm (gray bar) and 21.1 mm (closed bar) CaCl2 in place of EGTA, and E2P hydrolysis was followed (see typical examples with wild type and 5Gi-106/107 in the inset). The amounts of E2P formed with 32Pi at zero time are normalized to 100%. Solid lines show the least squares fit to a single exponential, and the rates thus obtained are shown in the main panel. In the absence of Ca2+, statistical significance of the mutant rate compared with the wild-type rate is shown above an open bar: *, p < 0.05; ‡, p < 0.01. In each of the mutants and the wild type, statistical significance of the rate in 3 and 20 mm Ca2+ compared with that in the absence of Ca2+ is shown above a gray bar and closed bar: V, p < 0.05; ↓, p < 0.01. B, the marked retardation and acceleration in the absence of Ca2+ (open bars in A) are visualized with α-carbon coloring, red and purple, respectively. Error bars, S.D.