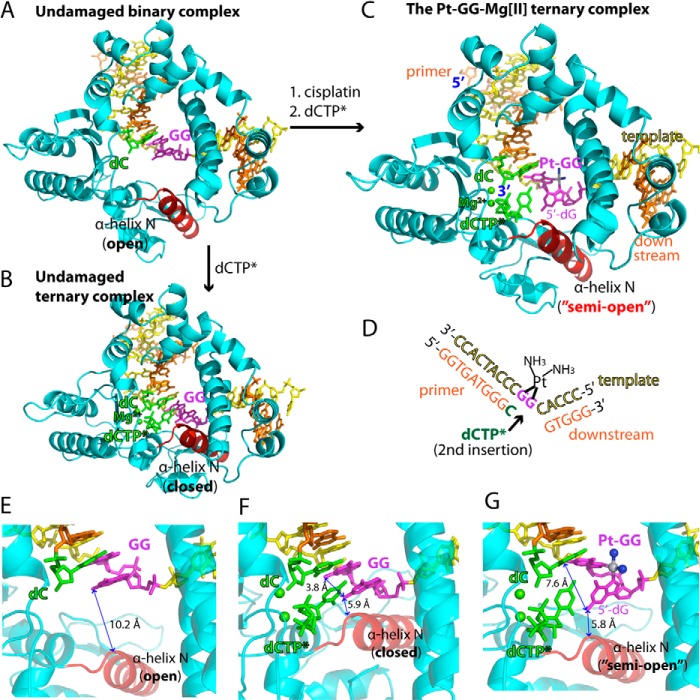
FIGURE 2.
Structures of the non-modified GG and the Pt-GG-Mg2+ complexes. A, overall structure of the unmodified GG gapped binary complex (Protein Data Bank code 4TUP). The template and primer strands are shown in yellow and orange, respectively. The α-helix N that contains the minor groove recognition amino acids is shown in red. The two guanines modified by cisplatin are shown in magenta. The primer terminus dC is shown in green. B, overall structure of the unmodified GG·dCTP* ternary complex (Protein Data Bank code 4TUQ). The incoming non-hydrolyzable dCTP* is shown in green. The active site Mg2+ ions are shown in green spheres. C, overall structure of the Pt-GG·dCTP*-Mg2+ ternary complex (Protein Data Bank code 4TUR). D, DNA sequences used for crystallographic studies. The site opposite the 5′-dG of the Pt-GG is indicated by an arrow. E, active site of the GG gapped binary complex structure. The distance between N2 of the templating dG and the α-helix N is indicated. F, active site of the GG·dCTP* ternary complex structure. The distance between N2(5′-dG) and N2(3′-dG) is indicated. G, active site of the Pt-GG·dCTP*-Mg2+ ternary complex structure. Platinum and NH3 of the Pt-GG lesion are shown in gray and blue spheres, respectively. The cisplatination of the GG increases the distance between N2(5′-dG) and N2(3′-dG).