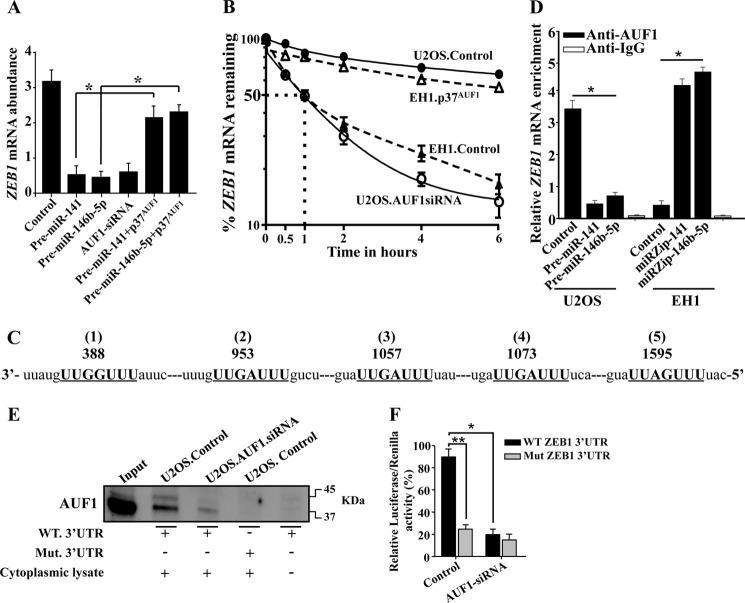
FIGURE 9.
AUF1 binds and stabilizes the ZEB1 mRNA. A, total RNA was prepared from U2OS cells expressing the indicated constructs and the level of the ZEB1 mRNA was assessed by qRT-PCR. Error bars, means ± S.D. *, p < 0.001. B, U2OS and EH1 cells expressing the indicated constructs were treated with actinomycin D and then reincubated for the indicated periods of time. Total RNA was extracted, and the remaining amount of the ZEB1 mRNA was assessed using qRT-PCR. The dotted lines indicate the ZEB1 mRNA half-life. Error bars, S.E. values of three different experiments. C, sequence alignment of human ZEB1 3′-UTR showing the AUF1 binding sites. D, RNAs bound to the AUF1 protein were isolated by immunoprecipitation from U2OS and EH1 cells expressing the indicated constructs using anti-AUF1 antibody or anti-IgG (control), and then the ZEB1 mRNA was amplified by qRT-PCR. Error bars, S.E. values of three different experiments; *, p ≤ 0.001. E, biotinylated ZEB1 3′-UTR bearing either wild type or mutated sequence of the second AUF1 binding site was incubated with cytoplasmic cellular lysate from the indicated cells, and the association of AUF1 with these RNAs was detected by immunoblotting using anti-AUF1 antibody. F, U2OS cells expressing AUF1 siRNA or a control plasmid were stably transfected with the luciferase reporter vector bearing either wild-type ZEB1 3′-UTR or a mutated sequence for one of the binding sites of AUF1 (residues 953–959). The reporter activity was assessed at 48 h post-transfection. Data (mean ± S.E., n = 4) are presented as a percentage change in reporter activity as compared with the negative control cells (*) or with the wild-type 3′-UTR (**). * and **, p < 0.00003.