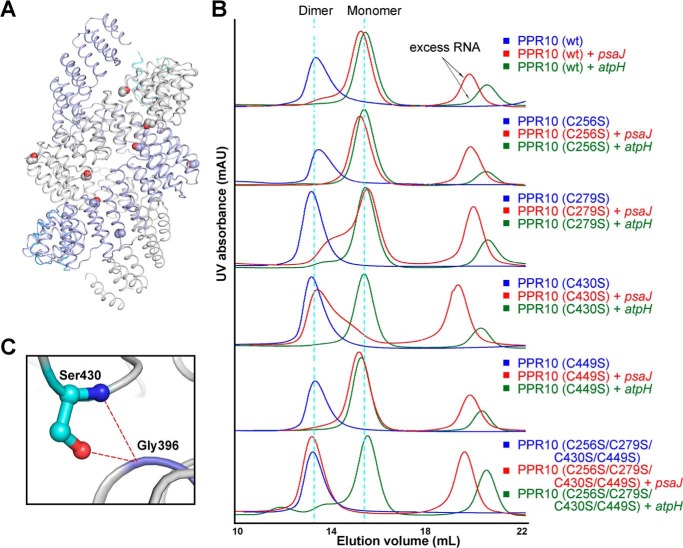
FIGURE 4.
Cysteine-to-serine mutations can affect the dimerization states of PPR10 in the presence of RNA. A, in the dimeric crystal structure of PPR10 (C256S/C279S/C430S/C449S), two protomers of PPR10 are colored light purple and gray with their NTDs colored blue and cyan. The four cysteine-to-serine mutation sites in each PPR protomer are highlighted as spheres. B, SEC analyses of wild type PPR10, PPR10 variants containing each single cysteine to serine mutation, and PPR10 with quadruple cysteine-to-serine mutations. The SEC analyses were performed in the absence and presence of psaJ or atpH RNA element. C, a close-up view of Ser-430. Ser-403 can form hydrogen bonds with Gly-396 from the adjacent repeat and is crucial for the dimerization of PPR10. The hydrogen bonds are shown as red dotted lines. mAU, milliabsorbance units.