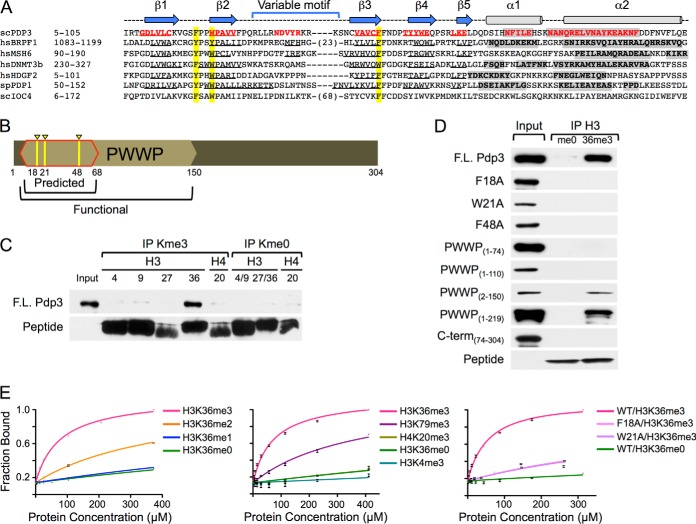
Fig. 2.
NuA3 specifically interacts with H3K36me3 through the PWWP domain of Pdp3. A, Clustal W alignment of PWWP domain-containing proteins. Beta sheets (arrows/underlined sequence) and alpha helices (cylinders/gray shading) are annotated. Aromatic cage residues are highlighted in yellow. See also Supplemental Fig. S1. B, Schematic representation of the Pdp3 protein. The predicted (red outline) and functional (tan hexagon) PWWP domains are annotated. Aromatic cage residues F18, W21, and F48 are highlighted in yellow. C, Peptide pull-down assays were performed with full-length HIS6-FLAG-Pdp3 and biotinylated histone peptides. Purified protein (input) and immunoprecipitated samples (IPs) were resolved by SDS-PAGE. Binding was monitored by western blotting. D, Peptide pull-down assays were performed with full-length HIS6-FLAG-Pdp3, mutants F18A, W21A, and F48A, truncations PWWP(1–74), PWWP(1–110), PWWP(2–150), PWWP(1–219), and C-term(74–304), and biotinylated histone peptides. Purified proteins (inputs) and immunoprecipitated samples (me0 and 36me3) were resolved by SDS-PAGE. Binding was monitored by western blotting. E, Fluorescence polarization assays were used to measure binding affinities of full-length S·tag-Pdp3 and mutants F18A and W21A to the indicated 5-FAM-labeled histone peptides. Error bars represent the S.D. of a representative experiment (n = 2) performed in triplicate.