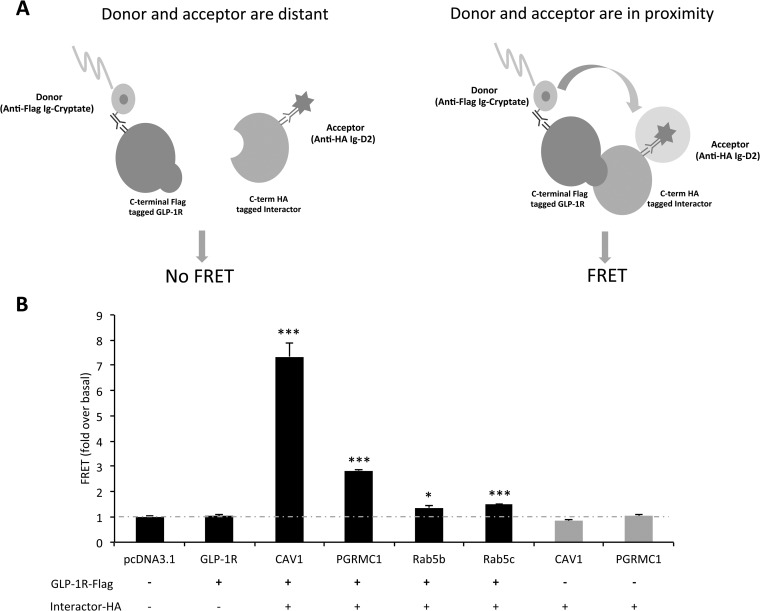
Fig. 4.
Validation of selected GLP-1R interactor by FRET. A, the principle of FRET for measuring protein–protein interaction. Biotinylated anti-Flag antibody linked to donor molecule (cryptate) and anti-HA antibody linked to acceptor molecule (d2) were incubated with cells expressing GLP-1R-Flag and interactor-HA. If the interactor of interest does not bind to GLP-1R-Flag, the donor and acceptor are not in close enough proximity to generate a FRET signal. If the interactor of interest binds to GLP-1R-Flag, two fluorophores (donor and acceptor) are in close enough proximity for fluorescent energy to resonate between them following excitation, and a FRET signal will be observed at 665 nm. Thus, HTRF technology combines the advantages of FRET and time-resolved measurement (low background) to measure protein–protein interactions. B, HTRF measurement of protein–protein interactions further confirmed the interaction between GLP-1R and CAV1, PGRMC1, Rab5b, and Rab5c (***p < 0.001, *p < 0.05, n = 3 per group).