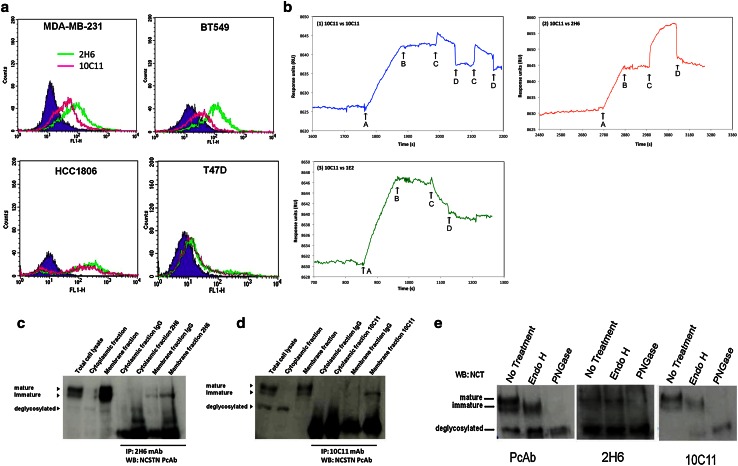
Fig. 1.
Binding properties of anti-nicastrin monoclonal antibodies in cell-based and non-cell-based assays. a Non-permeabilized MDA-MB-231, BT549, HCC1806 and T47D cells were incubated with the Rat IgG2b isotype control (blue), 2H6 (green) and 10C11 (pink) at 50 µg/ml. Anti-Rat-FITC conjugate was used as a secondary antibody. Histograms indicate a shift upon binding of the anti-NCSTN mAbs to NCSTN on cancer cells. b BIACore binding competition. Monoclonal antibody clone 10C11 was immobilised directly onto a CM5 chip and the antigen, NCSTN-Fc was flowed over at 1 mM concentration. NCSTN binding ‘start’ and ‘end’ is marked with ‘A’ and ‘B’, respectively. After a short period to allow for any weak dissociation, the test monoclonal antibodies (1) 10C11, (2) 2H6, and (3) 1E2 were flowed over as Fab fragments at 1 mM concentration. The start and ends are marked with ‘C’ and ‘D’, respectively. Additive binding (indicating a separate, non-overlapping epitope) is seen as a further increase in the sensogram signal, whereas competitive binding is seen as a dissociation curve. Clone 2H6 is shown to bind additively with clone 10C11. c, d Cell fractionation was performed using the ProteoExtract® Transmembrane Protein Extraction Kit (Novagen). Immunoprecipitation was done with 2H6 and 10C11 mAbs, and Western blots were probed using the nicastrin polyclonal antibody developed at Biogenes, GE against the nicastrin extracellular domain protein. e Nicastrin deglycosylation using EndoH and PNGase from whole cell lysates of MDA-MB-231 cells. Upon complete NCSTN deglycosylation with PNGase, 1E2, 2H6, 10C5 and 10C11 mAbs recognise the NCSTN non-glycosylated core protein at 80 kDa. This suggests that the mAbs bind peptide regions on the NCSTN ECD. Cell fractionation experiment showing that the 10C11 mAb is most specific for membrane bound Nicastrin. Immunopreciptation of NCSTN from both the cytoplasmic (cyto) and the membrane (memb) fractions of MDA-MB-231 cells using 10C11 and 2H6 MAb confirms that these mAbs binds membrane NCSTN. e Western blotting and glycosidase treatments of nicastrin in cellular extracts of MDA-MB-231 cells are shown. EndoH and PNGase treatments show the sensitivity of the various bands to deglycosylation. Native, fully deglycosylated nicastrin migrates at ∼ 80 kDa, which corresponds to the predicted molecular weight for the native protein. Treatment with PNGase produced a de-glycosylated form of nicastrin detected by mAbs