Figure 2.
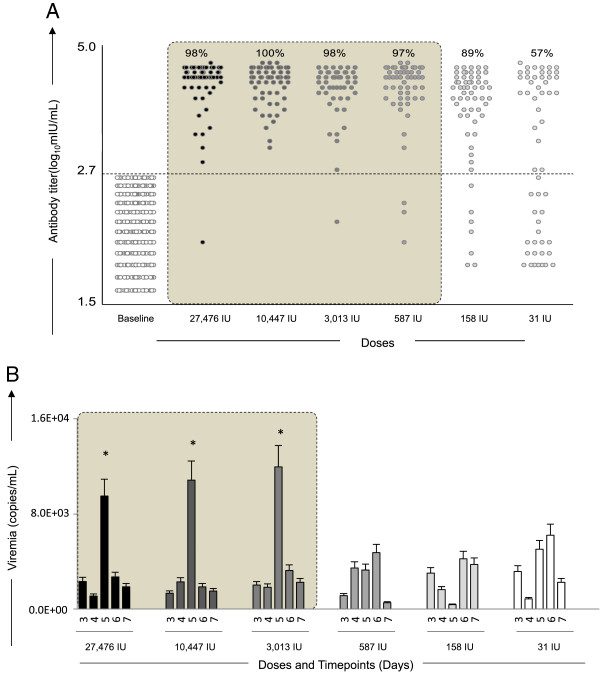
Immunogenicity and Viremia kinetics following 17DD-YF primary vaccination with different doses (27,476 IU-current; 10,447 IU; 3,013 IU; 587 IU; 158 IU and 31 IU). (A) Anti-YF neutralizing antibody titers (• = current dose and fades for subdoses) were measured by PRNT assay carried out 26–34 days (D30) after primary vaccination, as described in Methods. PRNT antibody titers are expressed in log10 mIU/mL and 2.7 log10 mIU/mL as the cut-off point to segregate seropositive from seronegative samples. Significant seropositivy rates (>95%) are highlighted by gray rectangle. (B) Viremia ( = current dose and fades for subdoses) was quantified by Real-time PCR at D3, D4, D5, D6 and D7 after primary vaccination as described in Methods. Viremia results are expressed in copies/mL. Kinetics profiles equivalent to current dose (27,476 IU) are highlighted by gray rectangle. The peaks of fold changes along the timeline were also taken into account as a relevant feature for equivalence assessment and highlighted by *.
= current dose and fades for subdoses) was quantified by Real-time PCR at D3, D4, D5, D6 and D7 after primary vaccination as described in Methods. Viremia results are expressed in copies/mL. Kinetics profiles equivalent to current dose (27,476 IU) are highlighted by gray rectangle. The peaks of fold changes along the timeline were also taken into account as a relevant feature for equivalence assessment and highlighted by *.
