Figure 1. Domain structure of the mTOR kinase and components of its protein complexes.
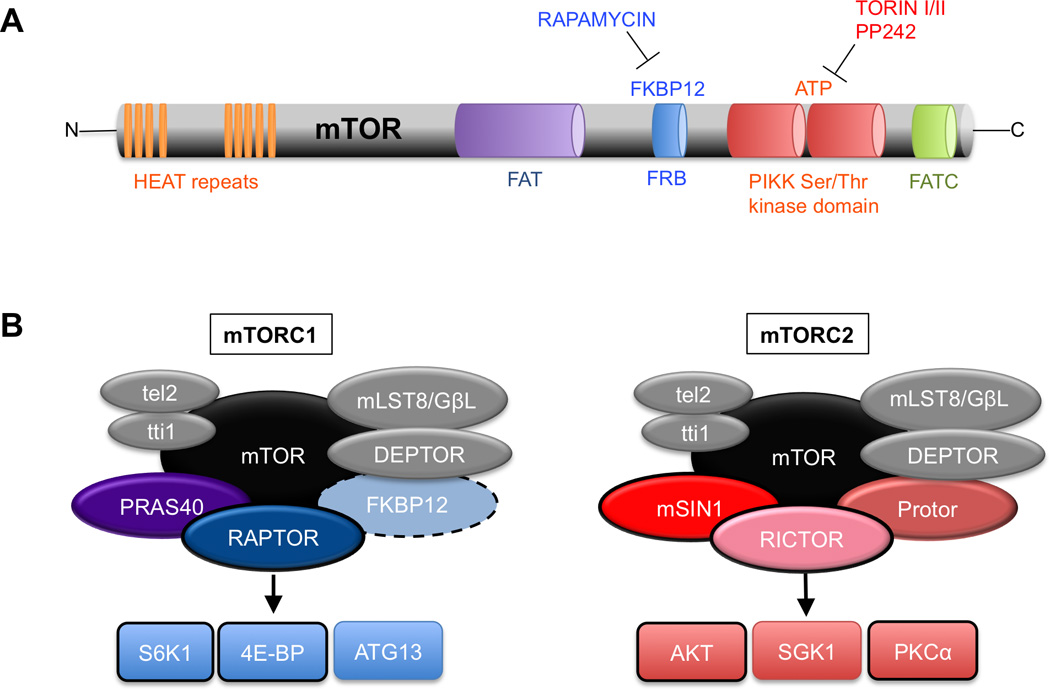
A. Domain organization of the mTOR kinase. HEAT (huntingtin, elongation factor 3, a subunit of phosphatase 2A and TOR1) repeats mediate protein interactions with Raptor, Rictor, and other proteins; FKBP12-rapamycin binding domain (FRB) is the site of rapamycin-mediated inhibition of mTORC1; The PIKK kinase domain contains the Ser/Thr catalytic activity and is the site of inhibition of kinase-site inhibitors such as Torin1 and Torin II, which inhibit both mTORC1 and mTORC2 activity; FATC = FRAP-ATM-TTRAP domain.
B. The components of mTORC1 and mTORC2. mTOR = mechanistic target of rapamycin; Raptor= scaffolding protein essential to mTORC1 activity and rapamycin sensitivity; PRAS40 = an inhibitor of mTORC1; DEPTOR = an inhibitor of mTORC1; mLST8/GβL = function unclear; Rictor = scaffold protein essential to mTORC2 function; mSIN1 = important for mTORC2 enzymatic activity toward AKT; Protor = mediates activity toward SGK. Black outlines indicate proteins that have been thoroughly examined in the nervous system. The dashed line around FKBP12 indicates that it is a non-obligate component of mTORC1.
