Figure 3.
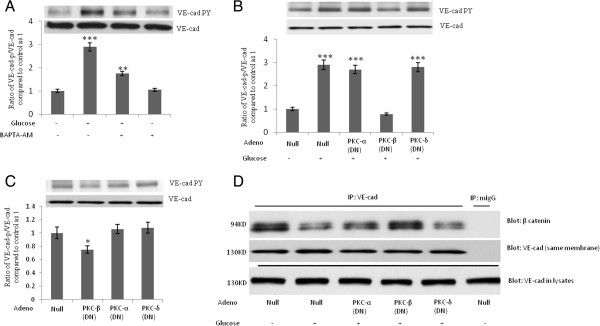
High-concentration glucose-induced tyrosine phosphorylation of VE-cad is mediated by protein kinase C (PKC)-b. A, Treatment of human umbilical vein endothelial cells (HUVECs) with 10 μM of cell-permeant intracellular calcium chelator, BAPTA-AM, for 2 h attenuated high-concentration glucose-induced tyrosine phosphorylation of VE-cad. HUVECs were first treated with 20 mM of glucose for 24 h. B, Overexpression of recombinant dominant-negative (DN)-PKC-β but not DN-PKC-α or DN-PKC-δ attenuated high-concentration glucose-induced tyrosine phosphorylation of VE-cad. C, Overexpression of DN-PKC-β but not DN-PKC-α or DN-PKC-δ reduced basal-level tyrosine phosphorylation of VE-cad. D, Overexpression of recombinant dominant-negative (DN)-PKC-β but not DN-PKC-α or DN-PKC-δ attenuated high-concentration glucose-induced dissociation of β-catenin from VE-cadherin complex. VE-cad antibody was used for immunoprecipitation, and β-catenin and VE-cad antibodies were used for the detection of each respective protein. mIgG (mouse non-immune IgG) was used as an immunoprecipitation control. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. Each experiment was independently performed 3 to 4 times.
