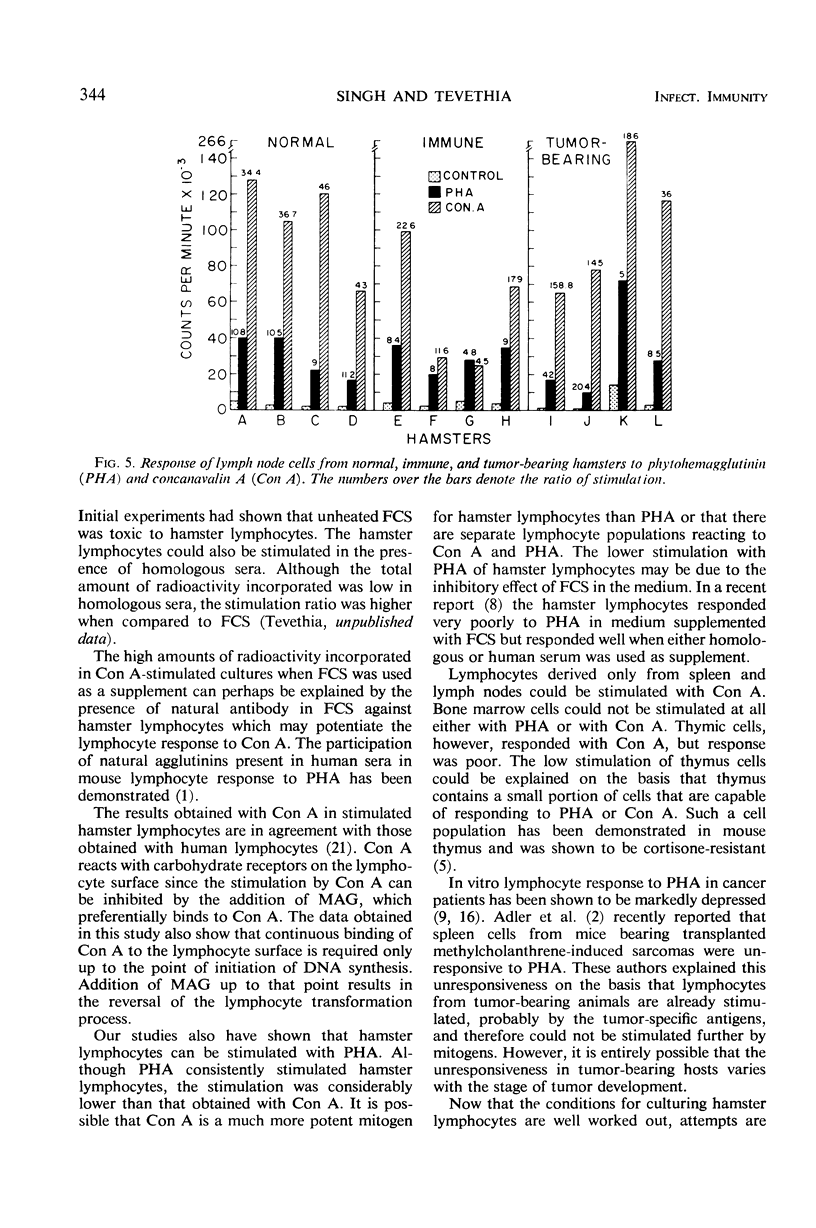
Abstract
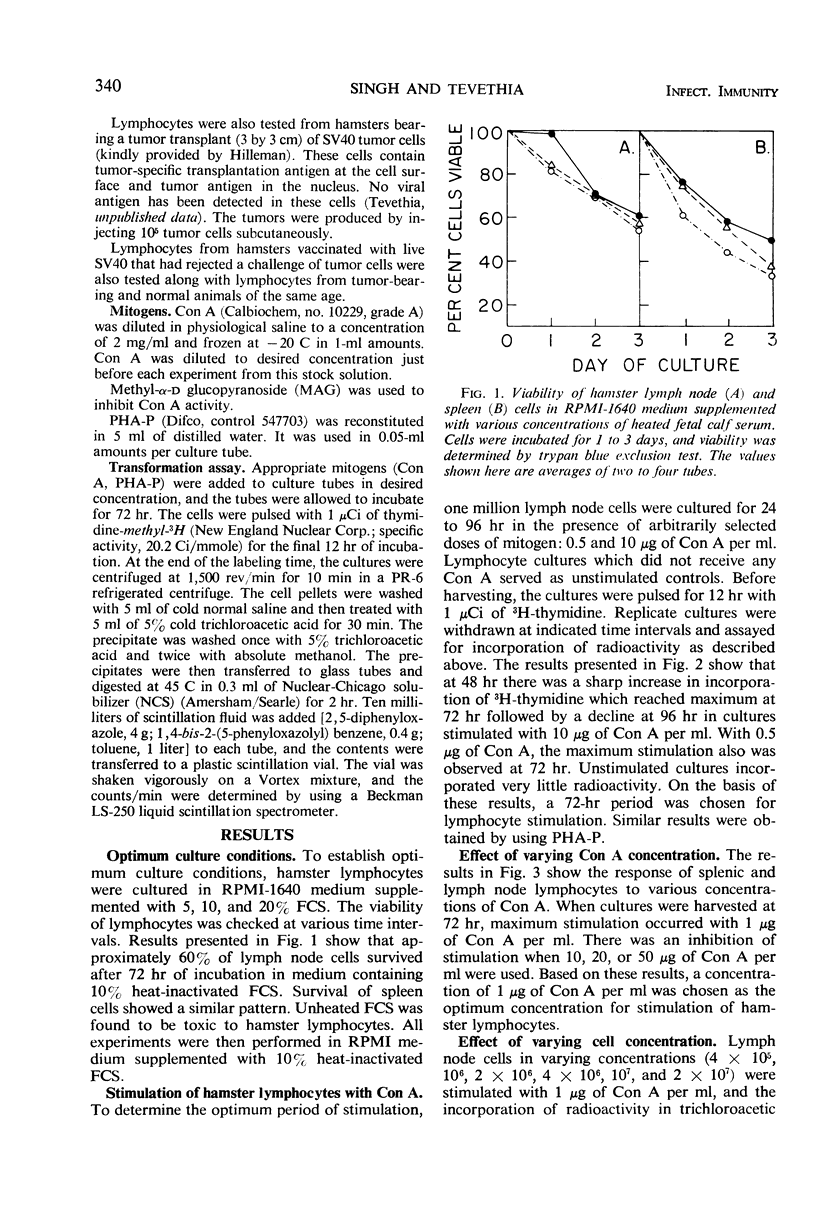
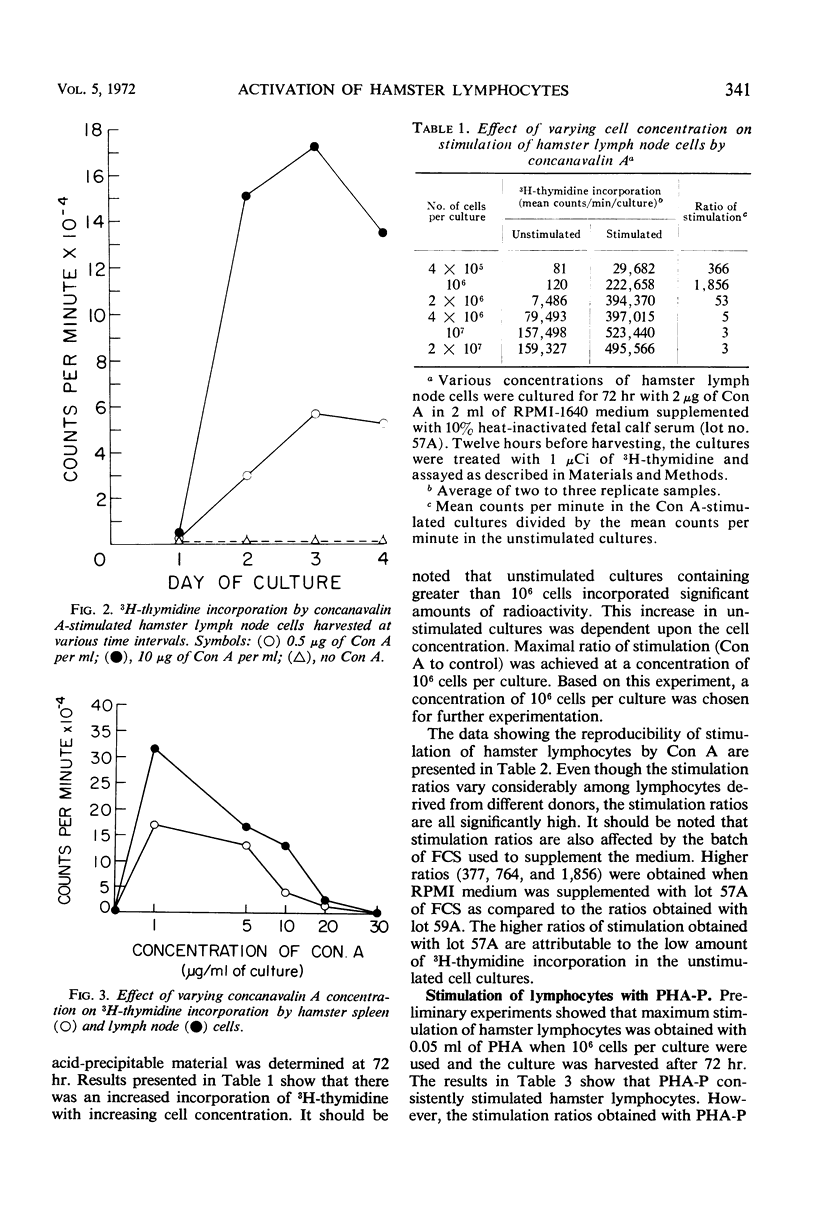
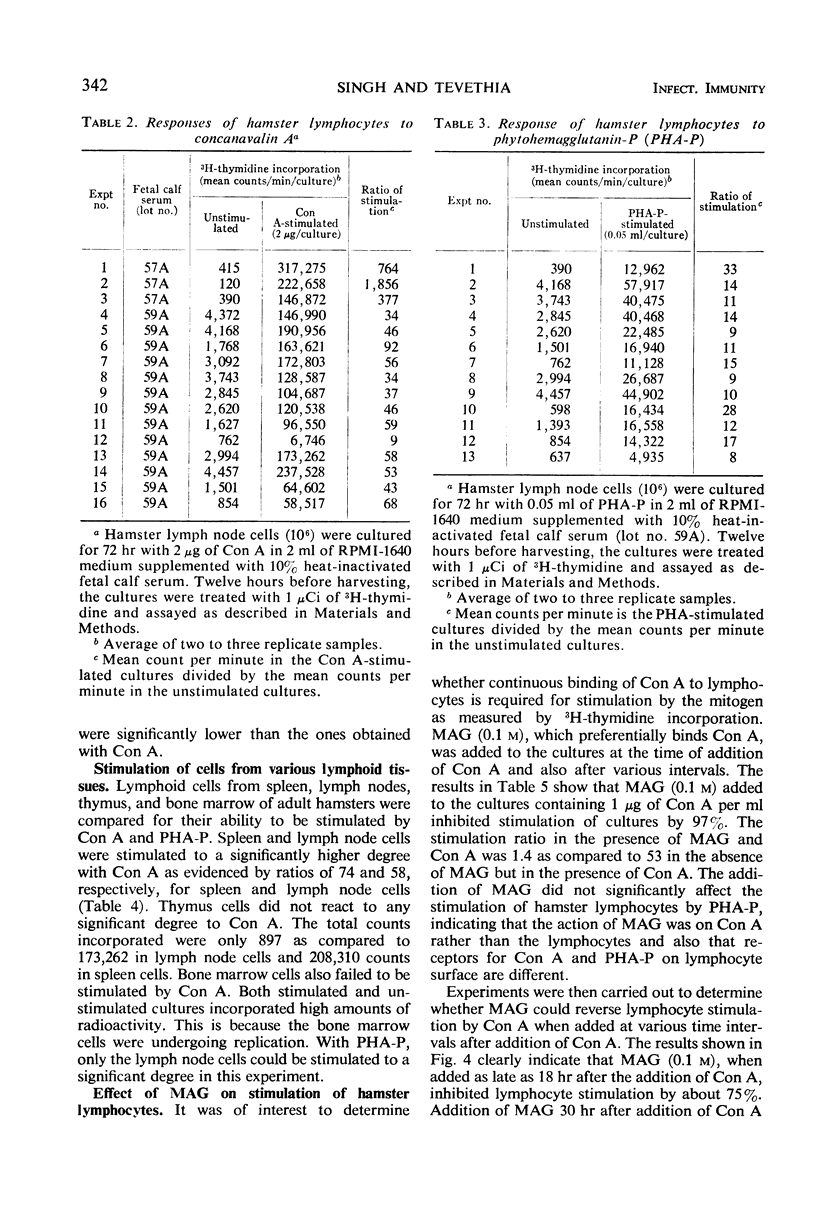
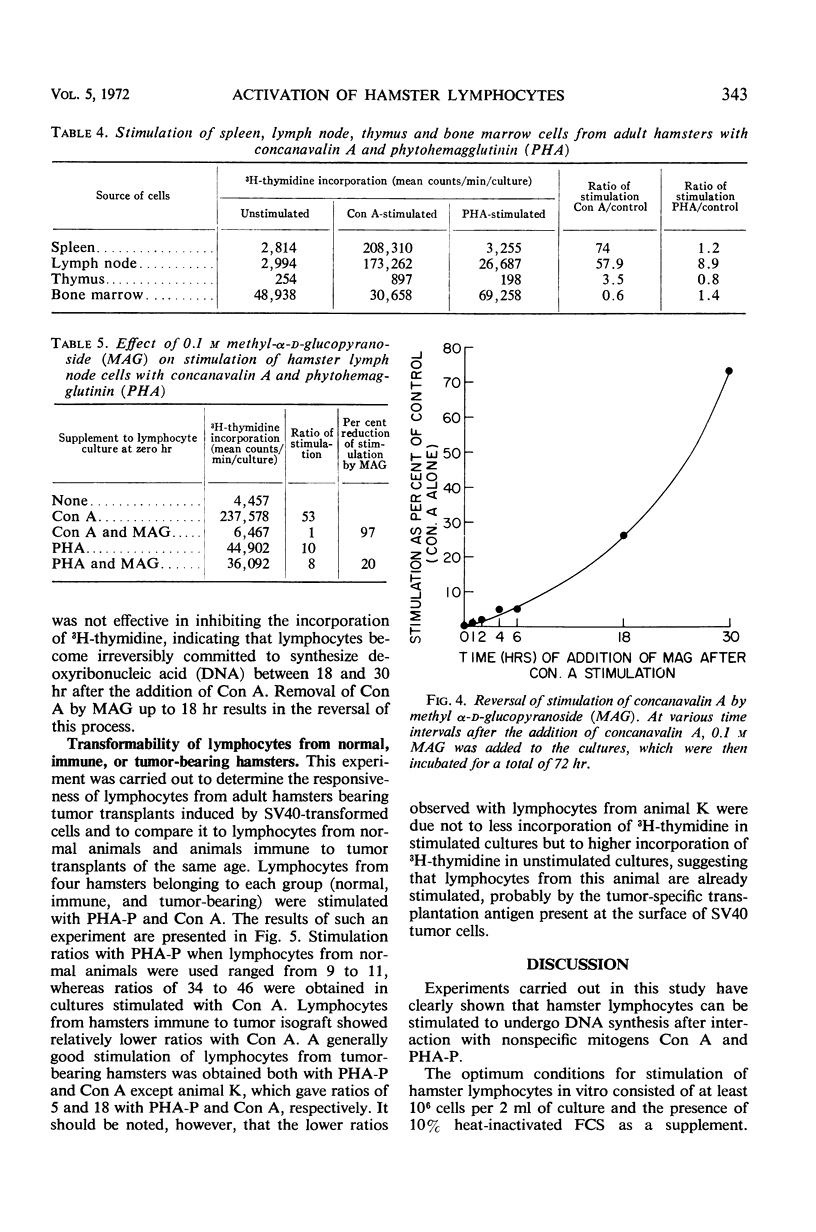
The in vitro response of hamster lymphocytes to concanavalin A (Con A) was investigated by using the 3H-thymidine incorporation assay, and the results obtained were compared to those with phytohemagglutinin (PHA-P). The optimum culture conditions for stimulation were obtained when one million lymph node cells were cultivated for 72 hr in 2 ml of RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum in the presence of either 2 μg of Con A or 0.05 ml of PHA-P. The average ratios of stimulation using lymph node cells with Con A and PHA-P were 227 and 14, respectively. Bone marrow and thymus cells responded very poorly or not at all. Methyl α-d-glucopyranoside, when added to the culture medium at the time of addition of Con A, completely inhibited stimulation. The lymphocyte stimulation could be reversed by addition of the sugar as late as 18 hr after the addition of Con A. The lymphocyte response from hamsters bearing tumors induced by simian virus 40 tumor cells and from hamsters immune to tumor transplants was comparable to the response of lymphocytes from healthy donors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Tumorigencity of simian papovavirus SV40 and of virus-transformed cells. J Natl Cancer Inst. 1963 Jun;30:1227–1265. [PubMed] [Google Scholar]
- Adler W. H., Takiguchi T., Marsh B., Smith R. T. Cellular recognition by mouse lymphocytes in vitro. I. Definition of a new technique and results of stimulation by phytohemagglutinin and specific antigens. J Exp Med. 1970 Jun 1;131(6):1049–1078. doi: 10.1084/jem.131.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACH F. H., HIRSCHHORN K. THE IN VITRO IMMUNE RESPONSE OF PERIPHERAL BLOOD LYMPHOCYTES. Semin Hematol. 1965 Jan;2:68–89. [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. In vitro stimulation of mouse thymus cells by PHA and allogeneic cells. Cell Immunol. 1971 Aug;2(4):285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- EDDY B. E., BORMAN G. S., GRUBBS G. E., YOUNG R. D. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology. 1962 May;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- EDDY B. E. SIMIAN VIRUS 40 (SV-40): AN ONCOGENIC VIRUS. Prog Exp Tumor Res. 1964;4:1–26. doi: 10.1159/000385971. [DOI] [PubMed] [Google Scholar]
- Fernald G. W., Metzgar R. S. In vitro studies of the response of hamster lymphocytes to phytohemagglutinin and antigenic stimulation. J Immunol. 1971 Aug;107(2):456–463. [PubMed] [Google Scholar]
- GIRARDI A. J., SWEET B. H., SLOTNICK V. B., HILLEMAN M. R. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV-40. Proc Soc Exp Biol Med. 1962 Mar;109:649–660. doi: 10.3181/00379727-109-27298. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E., Rogers E. A. Influence of cell density on response of leukocytes and column-purified lymphocytes to mitogenic agents. J Reticuloendothel Soc. 1970 May;7(5):567–586. [PubMed] [Google Scholar]
- Hersh E. M., Oppenheim J. J. Impaired in vitro lymphocyte transformation in Hodgkin's disease. N Engl J Med. 1965 Nov 4;273(19):1006–1012. doi: 10.1056/NEJM196511042731903. [DOI] [PubMed] [Google Scholar]
- Häyry P., Defendi V. I. Cultivation conditions and mixed lymphocyte interaction of mouse peripheral lymphocytes. Clin Exp Immunol. 1970 Mar;6(3):345–361. [PMC free article] [PubMed] [Google Scholar]
- Klein G. Tumor antigens. Annu Rev Microbiol. 1966;20:223–252. doi: 10.1146/annurev.mi.20.100166.001255. [DOI] [PubMed] [Google Scholar]
- Law L. W. Studies of the significance of tumor antigens in induction and repression of neoplastic diseases: presidential address. Cancer Res. 1969 Jan;29(1):1–21. [PubMed] [Google Scholar]
- Phillips S. M., Zweiman B. Characteristics of the in vitro response of guinea pig blood lymphocytes to PHA and antigen. J Immunol. 1970 Jul;105(1):204–214. [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Sell S., Rowe D. S., Gell P. G. Studies on rabbit lymphocytes in vitro. 3. Proteins, RNA, and DNA synthesis by lymphocyte cultures after stimulation with phytohaemagglutinin, with staphylococcal filtrate, with antiallotype serum, and with heterologous antiserum to rabbit whole serum. J Exp Med. 1965 Oct 1;122(4):823–839. doi: 10.1084/jem.122.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Quantitative studies on the mixed lymphocyte interaction in rats. I. Conditions and parameters of response. J Exp Med. 1967 Oct 1;126(4):625–654. doi: 10.1084/jem.126.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]


