Abstract
Microcapsules made of polyelectrolyte multilayers exhibit no or low toxicity, appropriate mechanical stability, variable controllable degradation and can incorporate remote release mechanisms triggered by various stimuli, making them well suited for targeted drug delivery to live cells. This study investigates interactions between microcapsules made of synthetic (i.e. polystyrenesulfonate sodium salt/polyallylamine hydrochloride) or natural (i.e. dextran sulfate/poly-l-arginine) polyelectrolyte and human umbilical vein endothelial cells with particular focus on the effect of the glycocalyx layer on the intake of microcapsules by endothelial cells. Neuraminidase cleaves N-acetyl neuraminic acid residues of glycoproteins and targets the sialic acid component of the glycocalyx on the cell membrane. Three-dimensional confocal images reveal that microcapsules, functionalized with neuraminidase, can be internalized by endothelial cells. Capsules without neuraminidase are blocked by the glycocalyx layer. Uptake of the microcapsules is most significant in the first 2 h. Following their internalization by endothelial cells, biodegradable DS/PArg capsules rupture by day 5; however, there is no obvious change in the shape and integrity of PSS/PAH capsules within the period of observation. Results from the study support our hypothesis that the glycocalyx functions as an endothelial barrier to cross-membrane movement of microcapsules. Neuraminidase-loaded microcapsules can enter endothelial cells by localized cleavage of glycocalyx components with minimum disruption of the glycocalyx layer and therefore have high potential to act as drug delivery vehicles to reach tissues beyond the endothelial barrier of blood vessels.
Keywords: endothelial glycocalyx, biodegradable microcapsules, neuraminidase, targeted drug delivery, transport across cell membrane
1. Introduction
There has been growing interest over the past decades in exploiting micro-sized vehicles to carry, target and confer controlled release of substances. However, delivering desired drugs by microcontainers to live cells remains a challenge in material science. Various drug delivery systems have been developed for specific biomedical applications. They deliver a defined quantity of a therapeutic payload to a specific target site or tissue, and can allow a controlled release rate, with or without an external trigger mechanism [1], such as ultrasound or light [2,3]. Micro-sized capsules, with a controlled delivery rate and pre-designated release sites, have been introduced as potential drug-containing vehicles [4–7]. The layer-by-layer (LbL) technique, based on the sequential adsorption of oppositely charged polymers on a charged substrate, has been developed to construct these microcapsules [8,9]. Oppositely charged proteins and polyelectrolytes are sequentially absorbed onto a charged template by electrostatic attraction [10], resulting in complex formation between polyanions and polycations of defined thickness. Empty capsules can be obtained after decomposing the template. The size and morphology of the microcapsules, the polyelectrolytes and thickness of the shell, the permeability and release-activation mechanism, can be controlled with the LbL method [11].
Recently, a number of natural polyelectrolytes and polypeptides have been used to fabricate biodegradable microcapsules. These capsules have been successfully internalized by several phagocytic cells such as cancer and immune cells [12,13]. So far, there has been no published report on the uptake of microcapsules by non-phagocytic cells. In this study, we investigate the interaction between microcapsules and vascular endothelial cells, and the role of the cell membrane glycocalyx layer in the internalization of microcapsules.
Endothelial cells line the luminal surface of blood vessels and are in direct contact with the circulating blood. They play a significant role in homeostasis, vascular regulation, blood cell activation and migration in physiological and pathological conditions [14,15]. The glycocalyx is a brush-like structure on the luminal surface of endothelial cells, and contains proteoglycans, glycosaminoglycans and glycoproteins [16]. The thickness of the glycocalyx layer ranges between a few hundred nanometres to a few micrometres [17]. Neuraminidase, an enzyme that cleaves N-acetyl neuraminic acid residues of glycoproteins and targets specifically the sialic acid component of the endothelial glycocalyx, is commonly used to manipulate the structure of the glycocalyx [18].
The aim of this study is to investigate the role of the glycocalyx on transmembrane movement of microcapsules in vitro using human umbilical vein endothelial cells (HUVECs). Microcapsules that are loaded with neuraminidase are fabricated in our laboratory. This enables localized degradation of the endothelial glycocalyx when microcapsules come into contact with the endothelial cell membrane. In the study, we investigate the encapsulation efficiency of neuraminidase and its release rate. Comparisons between neuraminidase-loaded microcapsules and control microcapsules were carried out. Furthermore, we investigated the degradation rate of DS/PArg microcapsules after their internalization in HUVECs.
2. Material and methods
Materials were purchased from Sigma-Aldrich: polystyrenesulfonate sodium salt (PSS), Mw = 70 k; polyallylamine hydrochloride (PAH), Mw = 56 k; dextran sulfate sodium salt from Leuconostoc spp. (DS), Mw = 100 k; poly-l-arginine (PArg), Mw = 15–70 k; neuraminidase from Clostridium perfringens; type V, lyophilized powder; ethylenediaminetetraacetic acid (EDTA); tetramethylrhodamine isothiocyanate–dextran (TRITC–dextran), Mw = 65–85 k; wheatgerm agglutinin (WGA–FITC); Hoechst 33342. From Invitrogen: cell tracker red CMTPX. All chemicals were used as received without additional purification.
2.1. Microcapsules preparation
Calcium carbonate (CaCO3) microparticles are more biocompatible than other templates for LbL encapsulation of proteins and enzymes [19]. The template core of CaCO3 was fabricated by mixing 0.33 M CaCl2 and equal volume of 0.33 M Na2CO3 solution into a beaker with stirring for 30 s at room temperature [20]. Subsequently, the solution was collected into tubes, and three centrifugation and washing steps were applied with Milli-Q water in order to remove residual salts and unreacted pieces. Spherical-shaped CaCO3 microparticles with a uniform, average diameter of approximately 4 µm were obtained. The porosity of CaCO3 particles provides a large surface area, and has the ability to capture macromolecules effectively. A method of pre-loading (see [19] for details) was performed for TRICT–dextran encapsulation. About 0.5 mg ml−1 of TRICT–dextran (red stain for visualization purpose) was premixed with CaCl2 or Na2CO3 aqueous solutions; protein was captured by co-precipitation during the process of growing CaCO3 microspheres. For neuraminidase-loaded microcapsules, 2 mg ml−1 neuraminidase was also encapsulated by the pre-loading method.
The alternating adsorption of polyelectrolytes onto the surface of CaCO3 particles was produced by immersing the particles into 2 mg ml−1, oppositely charged, polymer solutions, with 0.5 M sodium chloride at a pH between 5 and 7. The polyelectrolyte absorption time required was at least 15 min with continued shaking in order to fully cover the core particles surface. After each layer had been absorbed, core particles were centrifuged and washed with Milli-Q water three times to remove the uncoated polymer. Particle aggregation may occur during the process. A 10 s period in an ultrasonic bath was introduced to reduce the aggregation of the particles. After the desired number of capsule layers had been coated, CaCO3 template core particles were dissolved by adding 0.2 M EDTA at pH 7 three times. The capsules were then centrifuged and washed three times with Milli-Q water.
Previously, we have tried microcapsules with a different number of layers as a means to adjust the release rate of the encapsulated substance [21]. With more layers, the capsule wall is thicker and less permeable. Capsules also become more rigid, making them less favourable for cell uptake. In this study, four-layered capsules were fabricated and used, i.e. (PSS–PAH)2 and (DS–PArg)2 microcapsules. They were seen to have a uniform size and shape, with the average diameter of approximately 4 µm.
2.2. UV−vis spectroscopy
A UV−vis spectrophotometer (LAMBDA 950, Perkin-Elmer) was employed to measure neuraminidase concentration in an aqueous solution with UV–quartz glass cuvette. Intensity at 280 nm wavelength was used to measure neuraminidase concentration in an aqueous solution, and calibration curves were performed using the intensity of neuraminidase solutions of known concentration.
2.3. Zetasizer nano
Zeta potential measurements of capsules were carried out in water using a Zetasizer nano system (Malvern Instruments, Germany). The measured potential values are given in appendix.
2.4. Cell culture and cell proliferation
HUVECs (primary pooled) were commercially sourced (Lonza Cologne AG, Germany). They were thawed and cultured in the M199 medium (Gibco) in collagen type-I-coated flasks (5 µg ml−1) at 37°C and 5% CO2. The culture medium contains 10% fetal bovine serum, EC growth factor-β (1 ng ml−1), EC growth supplement from bovine neural extract (3 µg ml−1), thymidine (1.25 µg ml−1), heparin (10 µg ml−1), penicillin (100 U ml−1), streptomycin (100 mg ml−1). All supplements were from Sigma-Aldrich. The medium was changed every 2 days. After reaching 80% confluence, HUVECs were treated with 0.25% trypsin, containing 0.02% EDTA, and cells were split and re-seeded on collagen type-I-coated glass slides [22].
2.5. Immunofluorescence staining
HUVECs on coverslips were stained as follows: wheatgerm agglutinin (WGA–FITC) was used to bind to N-acetyl-d-glucosamine and sialic acid component of the glycocalyx; the endothelial cytoplasm was stained by cell tracker red CMTPX, and cell nuclei were stained using Hoechst 33342. Samples were briefly washed three times using the serum-free M199 medium before each staining step. WGA–FITC and cell tracker red were applied to live cells for 15 min at 37°C before Hoechst 33342 was applied for 10 min. Cells were washed and immersed in culture medium containing 10% serum immediately after staining, and were kept in the culture medium with serum during confocal observation.
2.6. Enzyme treatment using neuraminidase
Neuraminidase-treated samples were prepared according to the protocol by Barker et al. [18] as in previous studies [22,23]. HUVECs samples were gently washed with serum-free M199 medium twice before they were incubated with 1 mg ml−1 neuraminidase medium for 10 min at 37°C. They were then stained and observed under the confocal microscope.
2.7. Confocal laser-scanning fluorescence microscopy
Confocal laser-scanning fluorescence microscopy (CLSM) images were captured with a Leica TCS SP2 inverted confocal microscope system (Leica, Germany). It is equipped with a 63× oil immersion objective lens that has a numerical aperture of 1.20. Ar–ion (488 nm) and He–Ne (543 nm) lasers as well as ultraviolet–visible infrared light (350 nm) were used as excitation sources to the fluorophores stained in the sample.
3. Results and discussion
3.1. Cleavage of the glycocalyx by neuraminidase
Different enzymes are able to cleave different components of the glycocalyx. Protocols for enzymatic treatments have been studied in a number of previous investigations [18]. In this study, we first established that neuraminidase abolishes the glycocalyx layer on our cultured endothelial cells. Then, we investigated the interaction between microcapsules and HUVECs with and without the glycocalyx layer. Finally, we studied the interaction between neuraminidase-loaded microcapsules and HUVECs.
Figure 1 depicts three-dimensional confocal images of HUVECs from the glass slide to the top of the cell at a series of depths along the z-axis, with Δz = 250 nm. The main enface panel shows the fluorescent image in the x–y cross section at a given z-location. The two smaller panels reveal the structure along the x–z (bottom panel) and y–z cross sections (right side panel) as indicated by dashed lines in the enface image. Figure 1a is the control, where HUVECs have been cultured for 14 days. In the image, green is WGA–FITC, red is cytotracker and blue indicates cell nuclei. The green-stained glycocalyx layer is seen to have a thickness of several hundred nanometres to 1 μm. It is continuous and covers the surface of the endothelium. Following neuraminidase treatment, shown in figure 1b, there is hardly any green fluorescence in the image; giving evidence that neuraminidase cleaves away most of the glycocalyx layer from the HUVEC membrane.
Figure 1.
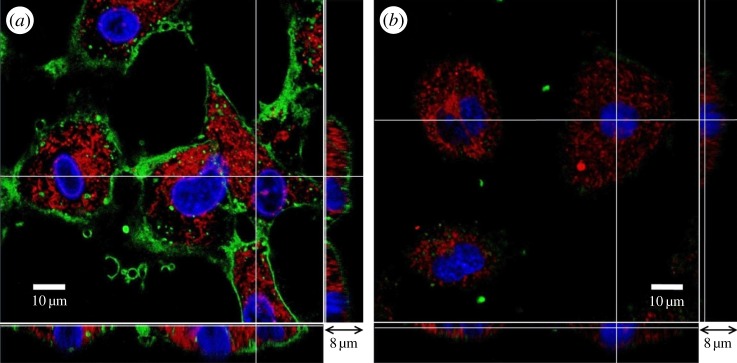
Three-dimensional confocal images of HUVECs. (a) Control, (b) neuraminidase-treated. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively. Scale bar in the main panel, 10 µm, and scale bar in the bottom and side panels, 8 µm.
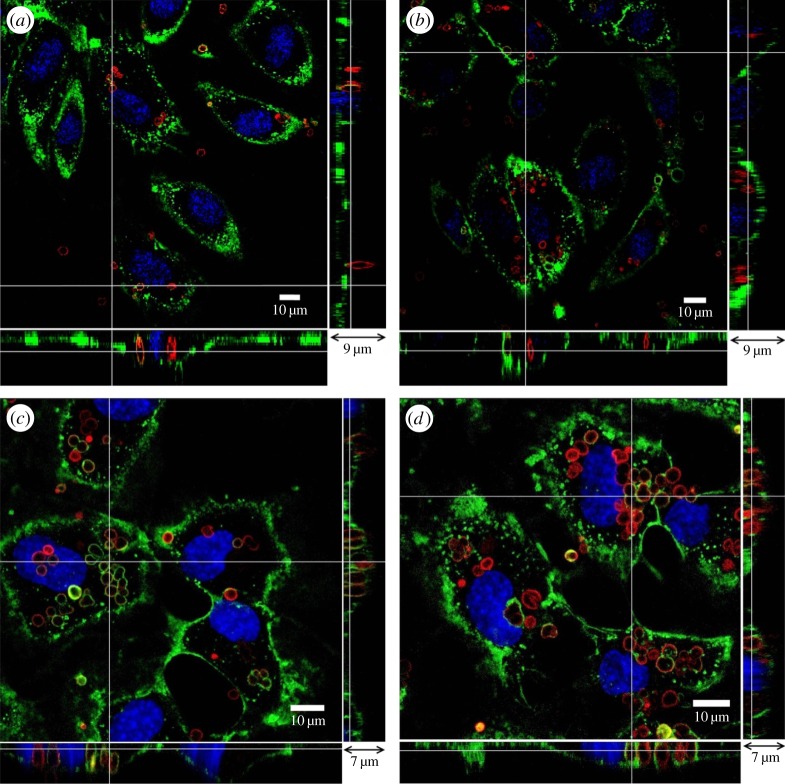
In our study, microcapsules were premixed with culture medium and incubated with HUVECs. It is therefore likely that the charge on microcapsule outermost layer will be changed by the culture medium through salt effects and protein adsorption. Results show that despite their initial difference in the charge property, both capsules exhibited negative charges in the cell culture medium (figure 12). Figure 2 shows the interaction between microcapsules and endothelial cells. In vitro cell-viability assays, such as the MTT (methylthiazol tetrazolium) test, showed no acute cell toxicity at moderate microcapsule concentrations [24]. In the entirety of this study, the ratio between the number of microcapsules and HUVECs was controlled at approximately 20 : 1. Further increase of the microcapsule concentration in the culture medium results in more capsules precipitated on the cell surface which may affect cell metabolism and viability [25,26]. For the control, shown in figure 2a, HUVECs were cultured for 14 days, and their membrane was covered with the glycocalyx layer. (PSS/PAH)2 microcapsules with TRITC–dextran (hence red fluorescence in the images) were added to the culture medium. Following 48 h incubation, there were a small number of microcapsules observed in the enface image. However, a closer look at the bottom and side panels in figure 2a revealed that these microcapsules were all on top of the endothelial glycocalyx and none had penetrated into the endothelial cells. Pre-treatment with 1 mg ml−1 neuraminidase, for 10 min at 37°C, abolished the glycocalyx layer on the HUVEC membrane (figure 2b). (PSS/PAH)2 microcapsules were added to the culture medium and incubated with the pre-treated HUVECs for 48 h. At the end of 48 h, part of the glycocalyx layer was recovered, but it was still patchy with little sign of green staining on the membrane above the cell nucleus. Microcapsules were observed inside the endothelial cells. This can be seen more clearly in the enlarged image in figure 2c, where red-stained microcapsules clustered around the cell nuclei. They were inside the cells and, in places, covered by the regrown glycocalyx layer. The x–z and y–z panels revealed that they were level with nuclei in the z-direction, rather than being on top of the cell membrane, as seen in the control. Previous study has shown that similar capsules enter the lysosome after their internalization [27]. The resolution of current confocal images does not allow us to look into this in detail.
Figure 2.
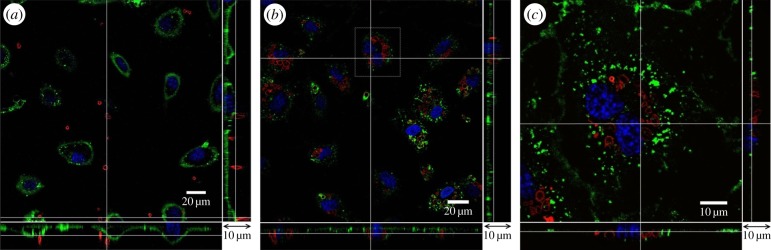
Interaction between microcapsules and endothelial cells. (a) Control, (b) endothelial cells were pre-treated with neuraminidase and then incubated with microcapsules for 48 h, (c) enlarged image of the highlighted area in panel (b). The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
These results demonstrate that the glycocalyx layer is capable of preventing microcapsules from entering the endothelial cells. Abolishing the glycocalyx layer enables the internalization of microcapsules by endothelial cells. However, if our goal is to use microcapsules as delivery vehicles into vascular endothelial cells, abolishing the entire glycocalyx layer in the vessel lumen can lead to severe pathological conditions, e.g. leaky vessels and oedema. It is, therefore, desirable and of practical relevance if we can functionalize microcapsules with neuraminidase, so that the cleavage of the glycocalyx layer happens only at locations where microcapsules come to contact with the endothelial cells.
3.2. Encapsulation of neuraminidase
Polyelectrolyte capsules are not completely impermeable to proteins. Protein release occurs according to capsule wall composition [28]. In our study, microcapsules (PSS/PAH)2 had four layers. Neuraminidase (4 mg) was co-precipitated with CaCO3 microparticles. Neuraminidase (0.836 mg) remained in the glass beaker with uncollected particles. During polyelectrolyte absorbing and capsule washing steps, there was a negligible amount of neuraminidase loss, and its concentration in the supernatant was too low to be detected by UV−vis spectroscopy. Neuraminidase (1.56 mg) was lost in the CaCO3 template decomposition step, called burst release. Therefore, approximately 21% and 39% of the neuraminidase was lost in the core coprecipitation and the dissolution steps, respectively, leaving 40% of the neuraminidase in capsules. The total number of capsules was quantified using haemocytometer counting chambers, and determined to be (7.8 ± 0.3) × 107. The amount of neuraminidase in a single microcapsule was therefore estimated to be approximately 20 pg.
To investigate the release rate of neuraminidase from (PSS/PAH)2 microcapsules, all microcapsules were placed in 2 ml Milli-Q water at room temperature. The supernatant was collected after 2, 24 and 72 h, and the surrounding water was fully refreshed at each time point. The concentration of neuraminidase in the collected supernatant was determined by the UV–vis spectrometer. Figure 3 shows the release rates of neuraminidase from (PSS/PAH)2 microcapsules. It is seen that in the first 2 h, neuraminidase was released at 12.82 µg h−1, but the value decreased to 10.36 µg h−1 over the next 22 h and then to 8.64 µg h−1 over the following 48 h. Protein encapsulation and release have been studied by a number of groups [21,29,30]. Our results are consistent with their findings that the release rate of proteins from polyelectrolyte microcapsules over the first hours is significantly higher than that over the following hours.
Figure 3.
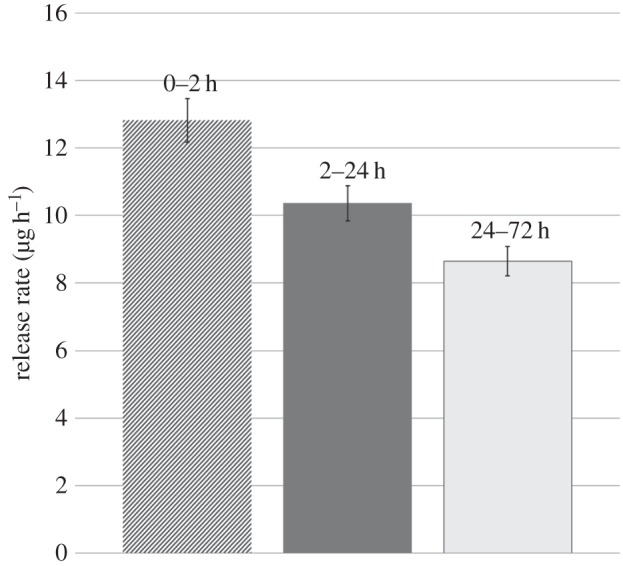
Neuraminidase release rate from (PSS/PAH)2 microcapsules at room temperature (number of experiments, n = 5).
3.3. Interaction between neuraminidase-loaded microcapsules and endothelial cells
(PSS/PAH)2 microcapsules were fabricated with neuraminidase as described before. They were divided equally into 20 portions and added to HUVECs with 2 ml culture medium. The number of microcapsules in each sample was quantified in the haemocytometer counting chamber. After 2 h incubation, the sample was washed three times with the culture medium. The number of microcapsules in the supernatant was again determined with a haemocytometer counting chamber. As for the control samples, similar experiments were carried out for (PSS/PAH)2 microcapsules without neuraminidase. Table 1 shows our data from experiments. For the control, most of the microcapsules were washed away, and only approximately 5% remained in the sample. For neuraminidase-loaded microcapsules, there were significant increases and approximately 30% of microcapsules had either adhered to or entered into vascular endothelial cells. Next, we set out to use confocal imaging to reveal the location of these microcapsules in relation to HUVECs.
Table 1.
Neuraminidase-loaded microcapsules show a significant increase in the number that adhere to or enter vascular endothelial cells after 2 h in the cell culture medium (number of experiments, n = 20).
| total number of capsules used in experiment (×106) | number of capsules washed away in supernatant (×106) | % of capsules entered or adhered to HUVECs (%) | |
|---|---|---|---|
| control | 4.07 ± 0.08 | 3.88 ± 0.21 | 4.67 |
| neuraminidase-loaded capsules | 3.93 ± 0.11 | 2.76 ± 0.17 | 29.77 |
During immunofluorescent staining steps, cultured HUVECs were briefly washed using the serum-free M199 medium nine times before imaging by a CLSM. All microcapsules suspended in the medium should be removed. Three-dimensional confocal images were taken from the glass slide to the top of the cell. In figure 4, the location of the microcapsules in relation to endothelial cells was observed after they were added to the cell culture medium. In the control group, shown in figure 4a, there were far fewer capsules in the enface image, as most of the microcapsules were washed away. The bottom and side panels in figure 4a revealed that the remaining capsules (red) were located on the glycocalyx layer (green) outside of the endothelial cells. By contrast, for neuraminidase-loaded microcapsules, shown in figure 4b, there were clusters of capsules near the cell nucleus in the enface image. From the bottom and side panels, we can see the capsules were inside the endothelial cell and localized to the same z-level as the nucleus. The glycocalyx layer was on top, covering the microcapsules.
Figure 4.
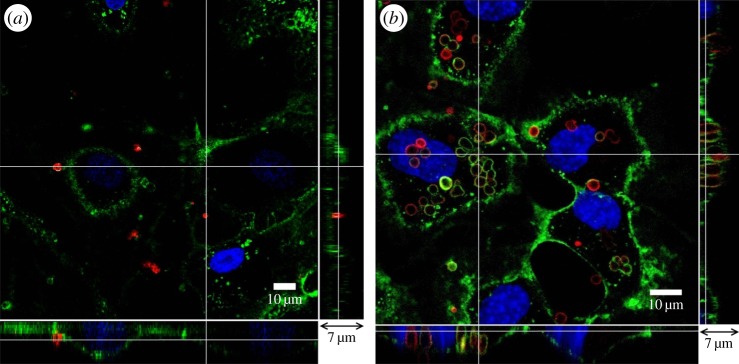
Interaction between (PSS/PAH)2 capsules and HUVECs after microcapsules were added to the culture medium for 2 h. (a) Control, (b) neuraminidase-loaded microcapsules. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
Note the difference between figure 4b and figure 2c. Although in both studies, microcapsules were able to enter the endothelial cells, in figure 2c, endothelial cells were pre-treated with neuraminidase to abolish their entire glycocalyx layer. The green stain represented the newly synthesized glycocalyx after 48 h culture. In figure 4b, neuraminidase-loaded microcapsules came to contact with the glycocalyx layer, cleaved the glycocalyx structure in their proximity and left most of the glycocalyx layer intact, hence the green stained glycocalyx layer in figure 4b remained well established. The process of a neuraminidase-loaded microcapsule entering a vascular endothelial cell is represented in a schematic drawing in figure 5.
Figure 5.
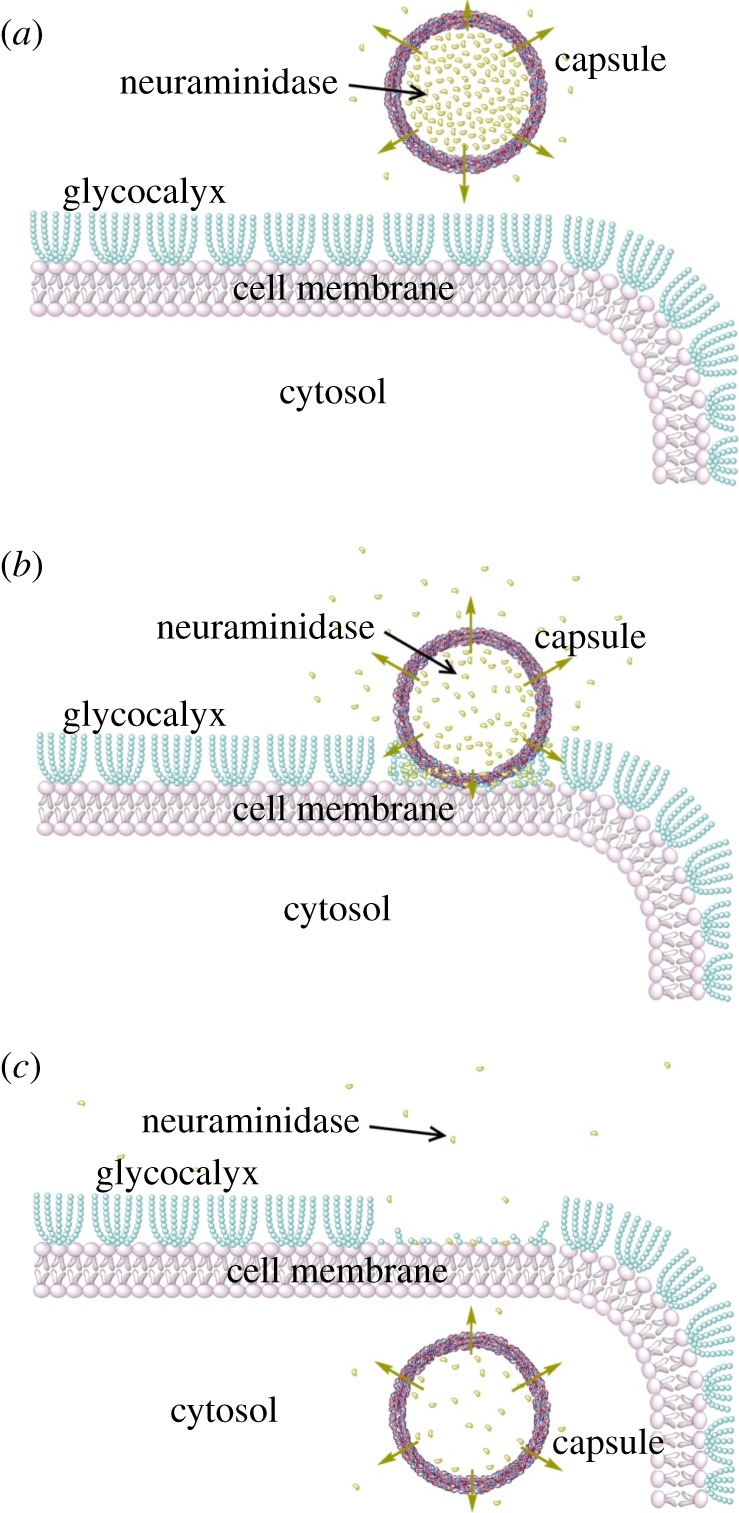
Schematic of the uptake of a neuraminidase-loaded microcapsule by a vascular endothelial cell. (a) Microcapsule is added to the culture medium and incubated with endothelial cells; (b) capsule comes to contact with the endothelial membrane. Neuraminidase released from the capsule cleaves the endothelial glycocalyx; (c) capsule is internalized by the endothelial cell.
3.4. Effect of time on the internalization of neuraminidase-loaded microcapsules
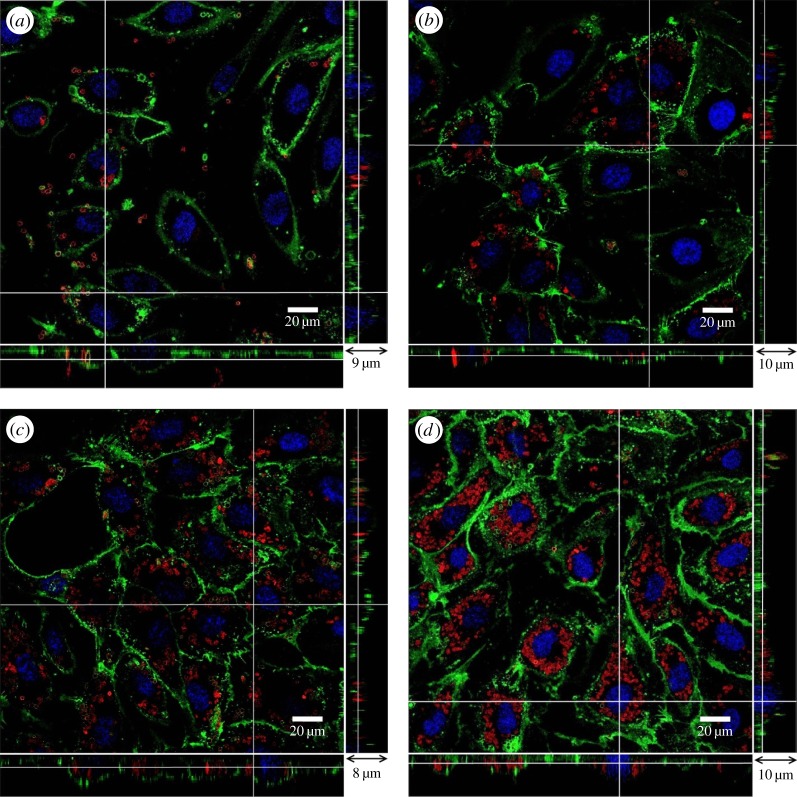
The time required for microcapsules to pass through the cell membrane is very important in drug delivery. In the following study, we investigate the uptake of neuraminidase-loaded (PSS/PAH)2 microcapsules by HUVECs at different time points. In figure 6, confocal images of capsules in HUVEC samples at 0.5, 1, 2 and 3 h are shown. Equal amounts of capsules were added to samples at the beginning of the experiment. At t = 0.5 h, shown in figure 6a, a number of microcapsules adhere to endothelial cells. The majority of these capsules were still above the glycocalyx layer, although a few have entered the cells. At t = 1 h (figure 6b), more capsules have made their way through the membrane into the cell. However, there were still many microcapsules above the endothelial glycocalyx layer. At t = 2 h (figure 6c), most capsules have passed through the cell membrane with only a few capsules outside the cell. Finally, at t = 3 h (figure 6d), we see a similar pattern to that observed in the t = 2 h sample, without further significant change. These results suggest that the approximate time required for HUVECs to internalize neuraminidase-loaded (PSS/PAH)2 microcapsules is approximately 2 h. This time will clearly be affected by the permeability of the capsules and the amount of neuraminidase encapsulated in them.
Figure 6.
Confocal images of neuraminidase-loaded (PSS/PAH)2 microcapsules in HUVEC samples at different time points. (a) t = 0.5 h; (b) t = 1 h; (c) t = 2 h and (d) t = 3 h. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
3.5. Stability of (PSS/PAH)2 microcapsules in human umbilical vein endothelial cells
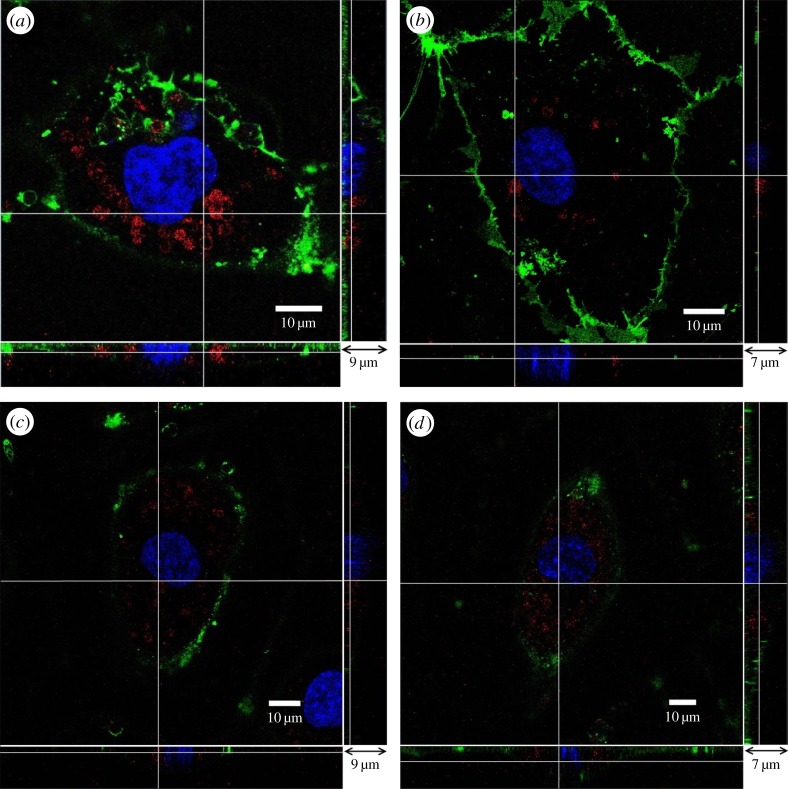
(PSS/PAH)2 capsules are categorized as non-biodegradable. Stability of neuraminidase-loaded microcapsules after their internalization by HUVECs was investigated in the following study. HUVEC samples containing (PSS/PAH)2 capsules in their cytoplasm were cultured for 7 days. At the end of day 7, HUVECs showed no difference to the control group, i.e. HUVECs cultured for the same length of time without capsules inside. In figure 7, we can see that (PSS/PAH)2 capsules retained their circular shape and integrity. The capsules remained in the cytoplasm, which can be seen in the bottom and side panels.
Figure 7.
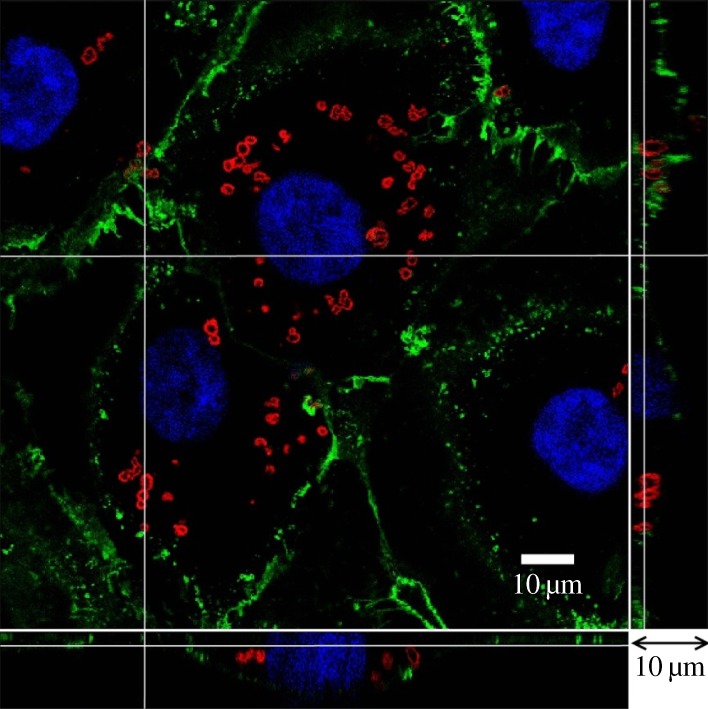
(PSS/PAH)2 capsules internalized by HUVECs retain their circular shape and integrity after 7 days. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
3.6. Biodegradable DS/PArg capsules
Although (PSS/PAH)2 microcapsules have excellent biocompatibility and can be functionalized with neuraminidase to enter vascular endothelial cells, they are not biodegradable. In drug delivery systems for therapeutic purposes and medical applications, the drug carrier needs not only be biocompatible, but also biodegradable over a reasonable period of time under physiological conditions. Polyelectrolytes microcapsules made of natural polysaccharides and polypeptides are known to be biodegradable, and once in phagocytic cells, they can degrade within hours [31]. De Geest et al. [13] developed polyelectrolyte microcapsules that could be degraded by intracellular proteases or hydrolytic enzymes. The dextran sulfate/poly-l-arginine (DS/PArg) microcapsules were susceptible to enzymatic degradation and gradually disintegrated over 60 h after being internalized by VERO-1 cancer cells. De Koker et al. [32,33] reported these microcapsules could also be degraded after incubation with bone-marrow-derived dendritic cells, and proposed macropinocytosis as the mechanism of uptake of capsules by the cells. Here, we used the same composition to make (DS/PArg)2 microcapsules and explore the capsule fate in non-phagocytic HUVECs.
Identical experiments to those presented above using (PSS/PAH)2 microcapsules were carried out on biodegradable (DS/PArg)2 microcapsules. First, we established the interaction between (DS/PArg)2 microcapsules and HUVECs with and without the glycocalyx layer. Figure 8 shows the results after (DS/PArg)2 microcapsules were added to HUVEC samples for 48 h. For the control group, in figure 8a, HUVECs have a well-developed glycocalyx layer, and (DS/PArg)2 microcapsules are observed on top of the glycocalyx outside the endothelial cells. For neuraminidase-pre-treated HUVECs, in figure 8b, clusters of (DS/PArg)2 microcapsules were observed near the nucleus of endothelial cells. The green stain represents the newly synthesized glycocalyx in the 48 h period. The bottom and side panels reveal these microcapsules are at the same z-location as the nucleus of the cell, and in places, they were underneath the glycocalyx layer. A close-up view is presented in figure 8c, where the location of the microcapsules inside the the endothelial cells can be seen more clearly.
Figure 8.
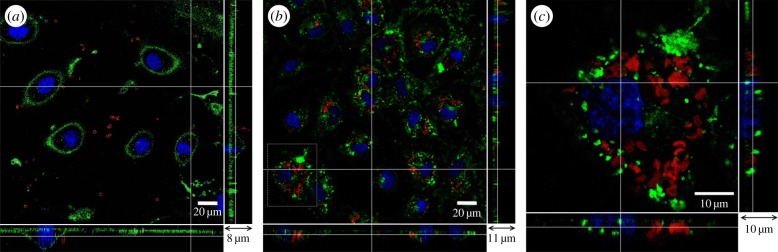
Interaction between (DS/PArg)2 microcapsules and HUVECs after 48 h incubation. (a) Control, capsules were seen on top of the glycocalyx layer, (b) endothelial cells were pre-treated with neuraminidase, and capsules were seen inside the cells clustered around the nucleus, (c) zoomed in view of the area highlighted in panel (b). The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
Internalization of neuraminidase-loaded (DS/PArg)2 microcapsules at different time points was studied at t = 0.5, 1, 2 and 3 h. As seen in figure 9, the number of internalized microcapsules increases with time. By t = 2 h, there are a large of number of capsules inside HUVECs, clustering in cytoplasm around nucleus. As time increases to t = 3 h, there is a further increase of capsules inside HUVECs, but the increase is less marked. A quantitative measure of the number of capsules in the endothelial cells at different times is given in figure 10.
Figure 9.
Internalization of neuraminidase-loaded (DS/PArg)2 microcapsules after they were added to the HUVEC culture medium for different lengths of time. (a) t = 0.5, (b) t = 1, (c) t = 2 and (d) t = 3 h. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
Figure 10.
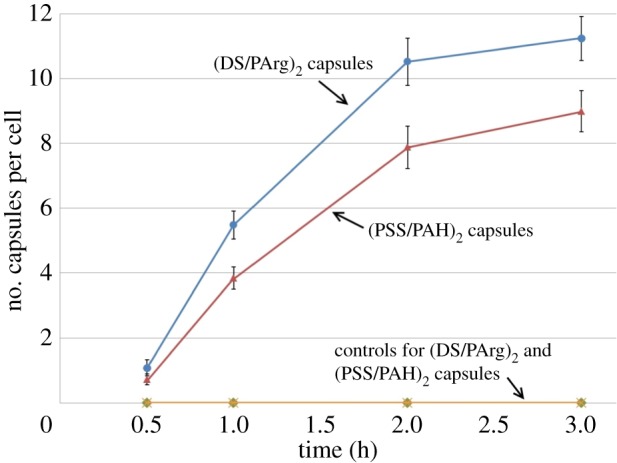
Number of internalized capsules per cell over time, following their addition to HUVEC samples. Every point in the results was based on data from five independent experiments. Each experimental replicate was the averaged value based on 20 cells. (Online version in colour.)
Figure 10 shows the relationship between the number of internalized capsules and the time after they are added to HUVEC samples. For both (PSS/PAH)2 and (DS/PArg)2 capsules, their presence in endothelial cells was heterogeneous. There are cells that take in many capsules, whereas the neighbouring cells have taken in few capsules. In our study, we chose two sampling areas randomly in an experiment, with approximately 20 cells being examined. The average number of capsules in a cell in an experiment was recorded. The experiment was repeated five times for each time point. For both (PSS/PAH)2 and (DS/PArg)2 capsules, the number inside the cell increases with the time. The increase in number is faster at earlier time points (i.e. up to t = 1 h) and slows down after 2 h. A higher number of biodegradable (DS/PArg)2 capsules are observed in cells than (PSS/PAH)2 capsules at all given time points. The results support the hypotheses that neuraminidase-loaded microcapsules release the enzyme to cleave the glycocalyx in the proximity of the capsules, leading to their internalization in vascular endothelial cells.
3.7. Degradation of (DS/PArg)2 capsules in human umbilical vein endothelial cells
To investigate the degradation of (DS/PArg)2 capsules after their internalization in HUVECs, we studied samples at different days. Figure 11 shows that (DS/PArg)2 capsules gradually disintegrate between 3 and 7 days. After 1 day in endothelial cells, as shown in figure 11a, capsules retain their circular shape and integrity. By 3 days, some of the capsules start to change their shape to appear as become discontinuous, patchy circles. By the end of 5 days, in figure 11c, most of the capsules have disintegrated to pieces and hardly any intact capsules can be seen inside endothelial cells. By the end of 7 days, as seen in figure 11d, microcapsules have completely degraded into pieces, with the red-coloured polyelectrolytes clouds and dots spread in the entire cytoplasm.
Figure 11.
(DS/PArg)2 microcapsules after different periods inside endothelial cell: (a) 1 day, (b) 3 days, (c) 5 days and (d) 7 days. The main panel shows the enface image at a given z-depth. The bottom and side panels show the x–z and y–z cross-sectional images, respectively.
4. Summary
In this work, we studied interactions between (PSS/PAH)2 and (DS/PArg)2 microcapsules and vascular endothelial cells in vitro. We first established that these capsules are blocked by the endothelial glycocalyx layer and cannot penetrate through the cell membrane into endothelial cells. When the glycocalyx layer is abolished by neuraminidase, endothelial cells are accessible to both (PSS/PAH)2 and (DS/PArg)2 capsules. Previous studies have found that similar capsules are easily incorporated by phagocytic cells, cancer cells and some primary cells in culture, but very inefficiently by primary hippocampal neurons in culture [34]. As the interface between the circulating blood and vessel wall, vascular endothelial cells are in direct contact with the blood. Their primary function is to form a barrier to keep the circulating cells and particles in the blood. Our results show that capsules do not enter endothelial cells under normal conditions, which is consistent with the physiological function of endothelial cells. However, if we cleave the glycocalyx layer on the cell membrane (using neuraminidase in this study), capsules can be internalized by the endothelial cells. Although the mechanisms involved in the uptake of capsules by endothelial cells are not yet fully understood, the endothelial glycocalyx layer plays an important role in the internalization of these capsules.
To minimize destruction of the endothelial glycocalyx layer while achieving cross cell membrane transport of the capsules, we encapsulated neuraminidase inside (PSS/PAH)2 and (DS/PArg)2 capsules. By controlling the permeability of capsules to neuraminidase, we achieve gradual release of the enzyme through the porous membrane of the capsule. The neuraminidase molecules surrounding the capsules cleave the glycocalyx layer to open a channel for the capsule to go through. Neuraminidase-loaded capsules can pass through the endothelial cell membrane and remain in the cytoplasm for many days with no apparent effect on cell viability. Of the two capsule types studied, (DS/PArg)2 capsules are biodegradable, which makes them a more appropriate drug-delivery candidate.
The ideal situation is to fabricate capsules that allow controlled release of neuraminidase and retain the cargo until capsules degrade after reaching the target site. This can be achieved if the size of the cargo is significantly bigger than that of the enzyme [35]. When the size of the drug is similar to that of the enzyme, there will be leakage of the drug before capsules enter the cell. We have fabricated capsules with specific targeting ability by functionalizing their surface [36]. Compared with the situation where the drug has to be administrated systemically and thus diluted, drug-carrying capsules can achieve significantly higher delivery efficiency.
The novelty of this study lies in the fact that we demonstrated the effect of the endothelial glycocalyx in the interaction between microcapsules and endothelial cells. Furthermore, we functionalized microcapsules by loading them with an enzyme to enable them to pass through the glycocalyx layer and achieve cell internalization. Results from our study show that microcapsules can be functionalized to work as delivery carriers not only into phagocytic cells, but also into other types of normal healthy cells. Vascular endothelial cells are chosen in this study due to the fact that these cells have the primary function of being the ‘barrier’ between the circulating blood and the tissue. Large particles, such as proteins and microcapsules, cannot permeate the endothelial lining in vivo. In conclusion, we have fabricated microcapsules that have high potential to act as drug delivery carriers with the ability to pass through the endothelial barrier of blood vessels into surrounding tissues.
Acknowledgement
W.L. and K.B. were funded by Queen Mary University of London PhD scholarships in 2009–2013 and 2008–2012, respectively.
Appendix A. Zeta potential measurement
Four-layered PSS–PAH capsules with either a PSS outermost layer or PAH outermost layer, i.e. (PSS–PAH)2 or (PAH–PSS)2 capsules, were mixed with culture medium for 12 h and washed three times with Milli-Q water, before their zeta potentials were measured. Figure 12 shows the results and their comparison to the control, i.e. before they were exposed to the cell culture medium. It is seen that for the control, zeta potentials for PAH and PSS outermost layered capsules are of +10.13 mV and −17 mV, respectively. However, after 12 h in the culture medium, their zeta potentials have changed to −5.5 and −8.97 mV. Despite their initial difference in charge, both capsules exhibited negative charges in the cell culture medium.
Figure 12.
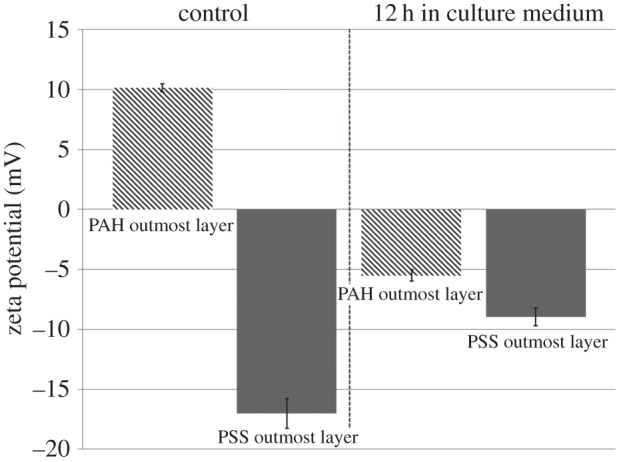
Zeta potentials of four-layered PSS–PAH microcapsules with either a PAH or PSS outmost layer after 12 h in the cell culture medium (n = 5).
Cell culture medium contains a variety of components and growth factors that can alter the charge of the capsule, including salts, such as NaCl (6800 mg l−1), KCl (400 mg l−1) and NaHCO3 (2200 mg l−1). Georgieva et al. [37] studied the influence of different salts on polyelectrolyte hollow microcapsules. They reported that the surface charge of capsules changed from initially positive to negative values after 24 h of incubation in each of the carbonate solutions, and demonstrated that the complete charge reversal of the PAH layer is caused by interaction of the carbonates with the weak polyelectrolyte PAH, which was confirmed by ITC measurements. This is consistent to our observation.
Funding statement
M.L. is supported by a Queen Mary University of London industrial PhD studentship in 2011–2015.
Reference
- 1.Langer R, Tirrell DA. 2004. Designing materials for biology and medicine. Nature 428, 487–492. ( 10.1038/nature02388) [DOI] [PubMed] [Google Scholar]
- 2.Pavlov AM, Saez V, Cobley A, Graves J, Sukhorukov GB, Mason TJ. 2011. Controlled protein release from microcapsules with composite shells using high frequency ultrasound: potential for in vivo medical use. Soft Matter 7, 4341–4347. ( 10.1039/c0sm01536a) [DOI] [Google Scholar]
- 3.Yi Q, Wen D, Sukhorukov GB. 2012. UV-crosslinkable multilayer microcapsules made of weak polyelectrolytes. Langmuir 28, 10 822–10 829. ( 10.1021/la300999b) [DOI] [PubMed] [Google Scholar]
- 4.De Geest BG, Sukhorukov GB, Möhwald H. 2009. The pros and cons of polyelectrolyte capsules in drug delivery. Expert Opin. Drug Deliv. 6, 614–624. ( 10.1517/17425240902980162) [DOI] [PubMed] [Google Scholar]
- 5.De Koker S, Lambrecht BN, Willart MA, van Kooyk Y, Grooten J, Vervaet C, Remon JP, De Geest BG. 2011. Designing polymeric particles for antigen delivery. Chem. Soc. Rev. 40, 320–339. ( 10.1039/b914943k) [DOI] [PubMed] [Google Scholar]
- 6.De Cock LJ, De Koker S, De Geest BG, Grooten J, Vervaet C, Remon JP, Sukhorukov GB, Antipina MN. 2010. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. 49, 6954–6973. ( 10.1002/anie.200906266) [DOI] [PubMed] [Google Scholar]
- 7.De Geest BG, Sukhorukov GB, Kreft O, Parak WJ, Skirtach AG, Demeester J, De Smedt SC, Hennink WE. 2009. Polyelectrolyte microcapsules for biomedical applications. Soft Matter 5, 282–291. ( 10.1039/b808262f) [DOI] [Google Scholar]
- 8.Sukhorukov GB, Donath E, Lichtenfeld H, Knippel E, Knippel M, Budde A, Möhwald H. 1998. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surfaces A, Physicochem. Eng. Aspect. 137, 253–266. ( 10.1016/S0927-7757(98)00213-1) [DOI] [Google Scholar]
- 9.Donath E, Sukhorukov GB, Caruso F, Davis SA, Möhwald H. 1998. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 37, 2201–2205. () [DOI] [PubMed] [Google Scholar]
- 10.Lvov Y, Ariga K, Ichinose I, Kunitake T. 1995. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 117, 6117–6123. ( 10.1021/ja00127a026) [DOI] [Google Scholar]
- 11.Sukhorukov GB, et al. 2007. Multifunctionalized polymer microcapsules: novel tools for biological and pharmacological applications. Small 3, 944–955. ( 10.1002/smll.200600622) [DOI] [PubMed] [Google Scholar]
- 12.Sukhorukov GB, et al. 2005. Nanoengineered polymer capsules: tools for detection, controlled delivery, and site-specific manipulation. Small 1, 194–200. ( 10.1002/smll.200400075) [DOI] [PubMed] [Google Scholar]
- 13.De Geest BG, Vandenbroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, Demeester J, De Smedt SC. 2006. Intracellularly degradable polyelectrolyte microcapsules. Adv. Mater. 18, 1005–1009. ( 10.1002/adma.200502128) [DOI] [Google Scholar]
- 14.Risau W. 1995. Differentiation of endothelium. FASEB J. 9, 926–933. [PubMed] [Google Scholar]
- 15.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. 1997. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91, 385–395. ( 10.1016/S0092-8674(00)80422-5) [DOI] [PubMed] [Google Scholar]
- 16.Pries A, Secomb T, Gaehtgens P. 2000. The endothelial surface layer. Pflügers Archiv. Eur. J. Physiol. 440, 653–666. ( 10.1007/s004240000307) [DOI] [PubMed] [Google Scholar]
- 17.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. 2001. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 136, 239–255. ( 10.1006/jsbi.2002.4441) [DOI] [PubMed] [Google Scholar]
- 18.Barker AL, Konopatskaya O, Neal CR, Macpherson JV, Whatmore JL, Winlove CP, Unwin PR, Shore AC. 2004. Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Phys. Chem. Chem. Phys. 6, 1006–1011. ( 10.1039/b312189e) [DOI] [Google Scholar]
- 19.Petrov AI, Volodkin DV, Sukhorukov GB. 2005. Protein: calcium carbonate coprecipitation: a tool for protein encapsulation. Biotechnol. Prog. 21, 918–925. ( 10.1021/bp0495825) [DOI] [PubMed] [Google Scholar]
- 20.Sukhorukov GB, Volodkin DV, Günther AM, Petrov AI, Shenoy DB, Möhwald H. 2004. Porous calcium carbonate microparticles as templates for encapsulation of bioactive compounds. J. Mater. Chem. 14, 2073–2081. ( 10.1039/b402617a) [DOI] [Google Scholar]
- 21.She Z, Antipina MN, Li J, Sukhorukov GB. 2010. Mechanism of protein release from polyelectrolyte multilayer microcapsules. Biomacromolecules 11, 1241–1247. ( 10.1021/bm901450r) [DOI] [PubMed] [Google Scholar]
- 22.Bai K, Wang W. 2012. Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J. R. Soc. Interface 9, 2290–2298. ( 10.1098/rsif.2011.0901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai K, Wang W. 2013. Shear stress-induced redistribution of the glycocalyx on endothelial cells in vitro. Biomech. Model. Mechanobiol. 13, 303–311. ( 10.1007/s10237-013-0502-3) [DOI] [PubMed] [Google Scholar]
- 24.An Z, Kavanoor K, Choy ML, Kaufman LJ. 2009. Polyelectrolyte microcapsule interactions with cells in two-and three-dimensional culture. Colloids Surfaces B, Biointerfaces 70, 114–123. ( 10.1016/j.colsurfb.2008.12.022) [DOI] [PubMed] [Google Scholar]
- 25.Kirchner C, Javier AM, Susha A, Rogach A, Kreft O, Sukhorukov G, Parak W. 2005. Cytotoxicity of nanoparticle-loaded polymer capsules. Talanta 67, 486–491. ( 10.1016/j.talanta.2005.06.042) [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Gao C, Shen J, Möhwald H. 2005. Multilayer microcapsules as anti-cancer drug delivery vehicle: deposition, sustained release, and in vitro bioactivity. Macromol. Biosci. 5, 1209–1219. ( 10.1002/mabi.200500176) [DOI] [PubMed] [Google Scholar]
- 27.Rivera_Gil P, Nazarenus M, Ashraf S, Parak WJ. 2012. pH-sensitive capsules as intracellular optical reporters for monitoring lysosomal pH changes upon stimulation. Small 8, 943–948. ( 10.1002/smll.201101780) [DOI] [PubMed] [Google Scholar]
- 28.She Z, Wang C, Li J, Sukhorukov GB, Antipina MN. 2012. Encapsulation of basic fibroblast growth factor by polyelectrolyte multilayer microcapsules and its controlled release for enhancing cell proliferation. Biomacromolecules 13, 2174–2180. ( 10.1021/bm3005879) [DOI] [PubMed] [Google Scholar]
- 29.Antipov AA, Sukhorukov GB, Donath E, Möhwald H. 2001. Sustained release properties of polyelectrolyte multilayer capsules. J. Phys. Chem. B 105, 2281–2284. ( 10.1021/jp002184+) [DOI] [Google Scholar]
- 30.De Geest BG, Sanders NN, Sukhorukov GB, Demeester J, De Smedt SC. 2007. Release mechanisms for polyelectrolyte capsules. Chem. Soc. Rev. 36, 636–649. ( 10.1039/b600460c) [DOI] [PubMed] [Google Scholar]
- 31.Rivera-Gil P, De Koker S, De Geest BG, Parak WJ. 2009. Intracellular processing of proteins mediated by biodegradable polyelectrolyte capsules. Nano Lett. 9, 4398–4402. ( 10.1021/nl902697j) [DOI] [PubMed] [Google Scholar]
- 32.De Koker S, De Geest BG, Singh SK, De Rycke R, Naessens T, Van Kooyk Y, Demeester J, De Smedt SC, Grooten J. 2009. Polyelectrolyte microcapsules as antigen delivery vehicles to dendritic cells: uptake, processing, and cross-presentation of encapsulated antigens. Angew. Chem. 121, 8637–8641. ( 10.1002/ange.200903769) [DOI] [PubMed] [Google Scholar]
- 33.De Koker S, De Geest BG, Cuvelier C, Ferdinande L, Deckers W, Hennink WE, De Smedt S, Mertens N. 2007. In vivo cellular uptake, degradation, and biocompatibility of polyelectrolyte microcapsules. Adv. Funct. Mater. 17, 3754–3763. ( 10.1002/adfm.200700416) [DOI] [Google Scholar]
- 34.Kastl L, et al. 2013. Multiple internalization pathways of polyelectrolyte multilayer capsules into mammalian cells. ACS Nano 7, 6605–6618. ( 10.1021/nn306032k) [DOI] [PubMed] [Google Scholar]
- 35.Borodina T, Markvicheva E, Kunizhev S, Möhwald H, Sukhorukov GB, Kreft O. 2007. Controlled release of DNA from self-degrading microcapsules. Macromol. Rapid Commun. 28, 1894–1899. ( 10.1002/marc.200700409) [DOI] [Google Scholar]
- 36.Deo DI, Gautrot JE, Sukhorukov GB, Wang W. 2014. Biofunctionalization of PEGylated microcapsules for exclusive binding to protein substrates. Biomacromolecules 15, 2555–2562. [DOI] [PubMed] [Google Scholar]
- 37.Georgieva R, Dimova R, Sukhorukov G, Ibarz G, Möhwald H. 2005. Influence of different salts on micro-sized polyelectrolyte hollow capsules. J. Mater. Chem. 15, 4301–4310. ( 10.1039/b507848b) [DOI] [Google Scholar]