Abstract
Background:
Prediabetes is a high-risk condition for type 2 diabetes mellitus. The growing prevalence of diabetes emphasizes on the necessity of concentrating on various strategies to prediabetes prevention and management. Probiotics as a group of functional foods might exert antidiabetic effects. This study aimed to assess the effects of probiotic administration on blood lipid profile and blood pressure in patients with prediabetes.
Methods:
This randomized controlled trial consisted of 60 prediabetic patients, aged 25-65 years old, that were randomly assigned to the intervention (receiving 500 mg probiotic capsules, n = 30) or control group (receiving placebo, n = 30) for 8-week period. Demographic and anthropometric data were collected at baseline. Blood samples were collected at baseline and after 8 weeks for biochemical measurements. Blood pressure was measured at the baseline an after 8 weeks of intervention. Data regarding dietary intakes and physical activity were also collected during the study. We used SPSS software version 16 (SPSS Inc. Chicago, USA) for data analyzing.
Results:
Probiotic supplementation did not contribute to significant changes in total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, Triglycerides (TG), TG/LDL and LDL/HDL ratios, after 8 weeks. After adjusting for potential confounders, HDL-cholesterol reduced significantly in the placebo group compared with probiotic group. Percent change in systolic blood pressure was significantly different in the probiotic group in comparison with a placebo group (–3.10 ± 2.22 vs. 3.24 ± 1.96, P = 0.01), although this significance did not exist anymore after adjusting for confounders (P > 0.05).
Conclusions:
Our study showed that probiotics did not have significant effects on lipid markers although they had positive effects on systolic blood pressure.
Keywords: Blood pressure, lipid, prediabetes, probiotic
INTRODUCTION
Diabetes mellitus (DM) type 2 is a metabolic disorder which can lead to the function failure of different organs, lipid profile disorders and elevated blood pressure.[1] 190 million subjects suffered from diabetes, in 2008 and according to the estimates; this number will reach 366 million, in 2030.[2] The prevalence of type 2 diabetes reached 7.7% among Iranian adults.[3]
Prediabetes is a high-risk condition with glycemic levels higher than normal range that do not meet diabetes cut-offs.[4] Its prevalence is higher than diabetes type 2.[5] Prevalence of prediabetes was 34.1% in 2007-2010[6] And more than 470 million people will become prediabetic until 2030.[4] Relative risk of cardiovascular diseases (CVD) is 2-4 times higher in diabetic patients in comparison with nondiabetic individuals.[7] Triglycerides (TG) to high-density lipoprotein (HDL) ratio is an important marker to identify insulin resistance in accompany with apparently healthy status, which reflect increased cardiometabolic disorders risk.[8] According to studies, incidence of hypertension is 1.5-3 times higher in diabetic patients compared with nondiabetics persons.[9]
Modifying lifestyle is recommended as an effective strategy to control pre-DM outcomes, and prevent or delay the development of diabetes among high-risk individuals.[10,11,12]
Probiotics are live microorganisms that can exert antidiabetic effects, improve glucose homeostasis and delay the progression of diabetes in different studies.[1,13,14,15,16] Dietary recommendations to both healthy and high-risk individuals such as prediabetic patients can be an effective strategy to prevent diabetes or its complications. Studies demonstrate that probiotic bacteria can improve glycemia and dyslipidemia.[13,17]
By means of this study, we aimed to evaluate the aforementioned health effects of probiotic supplementation on blood lipids and blood pressure in prediabetic individuals.
METHODS
Study design and participants
This study comprised 60 prediabetic patients, aged from 25 to 65 years old recruited from Endocrine and Metabolism Research Center affiliated with Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. Subjects with fasting plasma glucose concentrations of 100-125 mg/dL, 2 h glucose tolerance test levels of 140-200 mg/dL or both, for <2 months, were defined as eligible individuals. Subjects should have controlled state of glycaemia and lipid profile levels and were allowed to follow their prescribed medications during the study without changing their dosage. Exclusion criteria were defined as smoking; presence of kidney, liver, heart or respiratory disorders or inflammatory intestinal diseases, immune-deficiency disorders, taking antiinflammatory drugs and being in pregnancy or breast - feeding periods. To determine sample size, we used HbA1c as our main marker in another study, which is under review. Sample size was determined based on the primary information obtained from the study by Ejtahed et al. using following formula: 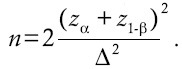 [18] To detect an effect size of 0.7, power of 80% and one-tailed significance level (α) of 0.05, 50 participants (25 participants for each group), were adequate. For expected dropout rate, we increased sample size to 60 (30 per group).[19] The present study was a double-blinded, randomized, placebo-controlled clinical trial. Volunteers were randomly assigned to take probiotic or placebo capsules. The randomization process in our study was conducted using blocks of size 2; we matched patients of each block based on age and sex. Subjects were instructed to keep their capsules under refrigeration and take each capsule once a day, after lunch for a period of 8 weeks. We also asked patients to maintain their usual dietary habits and lifestyle, without any medication change.
[18] To detect an effect size of 0.7, power of 80% and one-tailed significance level (α) of 0.05, 50 participants (25 participants for each group), were adequate. For expected dropout rate, we increased sample size to 60 (30 per group).[19] The present study was a double-blinded, randomized, placebo-controlled clinical trial. Volunteers were randomly assigned to take probiotic or placebo capsules. The randomization process in our study was conducted using blocks of size 2; we matched patients of each block based on age and sex. Subjects were instructed to keep their capsules under refrigeration and take each capsule once a day, after lunch for a period of 8 weeks. We also asked patients to maintain their usual dietary habits and lifestyle, without any medication change.
Intervention group were asked to take one 500 mg probiotic capsule every day for 8 weeks. Probiotic capsules contained 7 × 109 colony forming unit (CFU) Lactobacillus Casei, 2 × 109 CFU Lactobacillus Acidophilus, 1.5 × 109 CFU Lactobacillus Rhamnosus, 2 × 108 CFU lactobacillus Bulgaricus, 2 × 1010 CFU Bifidobacterium Breve, 7 × 109 CFU Bifidobacterium Longum, 1.5 × 1010 CFU Streptococcus Thermophilus as well as other ingredients such as fructooligosaccharide (as prebiotic), B group vitamins, maltodextrin, lactose and magnesium stearate (Familact, Zisttakhmir Co, Tehran, Iran). Control group received identical capsules containing starch. In this double-blinded study, the allocation of intervention or control group was concealed, and a nonaware person distributed probiotic and placebo capsules in identical containers, and neither the researchers nor the subjects were aware of the treatment assignments. Study participants received 56 capsules during the study period; 28 capsules in the beginning for the first 8 weeks of trial and 28 4 weeks later (in the middle of the study) for the rest 4 weeks of the study.
We weekly interviewed participants by telephone calls to monitor their compliance. An expert dietitian kept in touch with subjects to answer any possible questions.
Procedures and variable assessment
Demographic and anthropometric characteristics were measured at baseline. Body composition analyzer (Jawon Medical Company, Korea) measured weight while subjects were with light clothes and bare feet and hands, with 0.1 kg precision. Height was measured using a stadiometer with 0.5 cm precision in a normal standing position without shoes (Seca, Hamburg, Germany). body mass index (BMI) was then calculated by dividing body weight (kg) by height squared (m2). Physical activity levels were estimated through daily physical activity records completed in the beginning, in the middle and end of the intervention. Physical activity levels were calculated as metabolic equivalents/day.
An expert dietitian took three 24 h dietary recalls in the beginning, in the middle and at the end of the study. Nutritionist 4 software then calculated participants’ intake of specific nutrients.
Blood samples were collected after 12 h overnight fasting at the beginning and after 8 weeks. Samples were clotted in a 5-10 min period and then centrifuged at 3500 g for 10 min. Serum total cholesterol (TC) and TG levels were measured by enzymatic and colorimetric methods with Parsazmoon kits (Parsazmoon, Karaj, Iran). To measure serum HDL, other lipoproteins were blocked by antibodies, and HDL was specifically determined by enzymatic methods (Parsazmoon, Karaj, Iran). Low-density lipoprotein (LDL) was calculated by means of Friedewald formula.[20] All the biochemical measurements were done in the laboratory of IUMS, Isfahan, Iran.
Participants’ systolic and diastolic blood pressures were measured at the first day and last day of the intervention using a mercury sphygmomanometer, in accordance with American Heart Association protocols.[21] We asked patients to have a 10 min rest before measurement of their blood pressure.
Ethics Committee of IUMS approved the research process (No. 392157), and written informed consent was obtained from all of the participants prior to commencement of the study (Trial had been registered in the Iranian registry of clinical trials, available at: http://www.irct.ir, identifier: IRCT2013022411763N5).
Statistical analysis
We analyzed data using Statistical Package for Social Sciences (SPSS), version 16 (SPSS Inc., Chicago, USA) and numeric variables were expressed as mean ± standard errors (SEs). Smirnov-Kolmogorov tests were used to test normality of distribution of variables. For variables that did not follow the normal distribution, log transformation was performed. However, the results were not different in comparison with data analyzed before transformation. Background characteristics including weight, age, sex, drug usage, disease history and diet intakes of two groups were compared, using independent samples t-tests and Chi-square tests for quantitative and categorical variables, respectively. Changes in TC, HDL-cholesterol, LDL-cholesterol, TG, TG/LDL-C, TG/HDL-C and HDL-C/LDL-C levels, as well as patients’ systolic and diastolic blood pressures between the beginning and end of the trial were compared using paired samples t-tests.[22] Differences of percentage change of variables between two groups were assessed using multivariable analysis of variance. By using multivariable analysis of covariance, we adjusted potential confounders that differed between study groups at baseline, significantly. Results with P < 0.05 were considered to be statistically significant levels.
RESULTS
In this study, five patients were excluded from statistical analysis because of following results: Two patients were not interested in completing the rest of study, one subject had to change her drugs and start taking new supplements, one had nose surgery and one subject did not show up for final measurement and sampling. Data for 55 patients who completed the study entered in the analysis. Figure 1 shows a flow chart of participants. Baseline characteristics of study groups are presented in Table 1. Mean ± SE weight, age, and BMI of study groups were 76.15 ± 1.90 kg, 50.71 ± 1.01, 29.27 ± 0.56 kg/cm2, respectively. 70.4% of intervention subjects and 76% of the control group were men, which did not significantly differ between the groups (P > 0.05). There were no significant differences in demographic and anthropometric properties as well as drug/supplement use and medical history between two groups except for taking antihypertensive drug that was significantly different between study groups (P = 0.03). Physical activity levels were also not significantly different between the groups. Based on dietary recalls, study groups did not have any significant differences in overall dietary intakes throughout the study, [Table 2]. Probiotic administration led to nonsignificant reductions in TG/LDL and LDL/HDL. HDL-c was reduced in both probiotic and placebo groups although this reduction was very slight in probiotic group [Table 3]. After adjusting for potential confounder variables with significant baseline difference, the placebo group showed marginally significantly higher reduction in HDL-c compared with probiotic group (P = 0.06). Percent change in TG/HDL ratio was significantly different in the treatment group in comparison with the control group, too. Moreover, the placebo group showed a significant increase in TG/HDL ratio in comparison with treatment group before adjusting for confounders.
Figure 1.
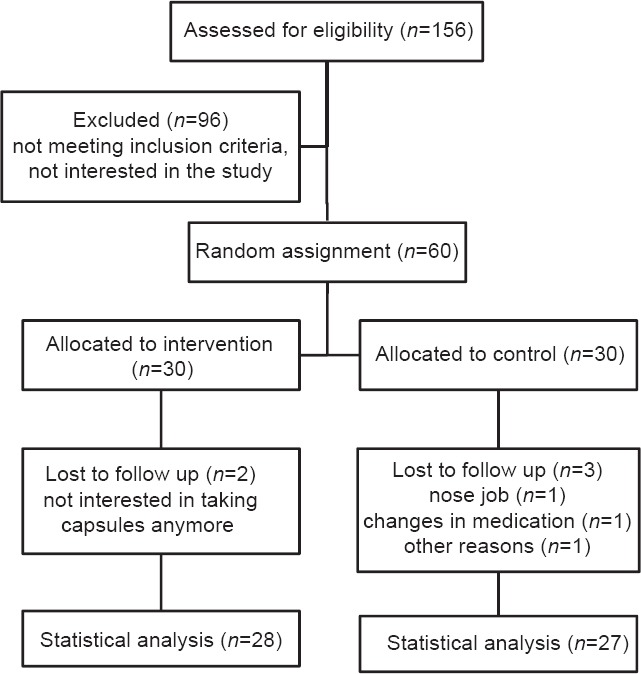
Overview of patients flow. Individuals in the intervention group received one probiotic capsule, every day for 8 weeks, individuals in control group received placebo in the same manner
Table 1.
Baseline characteristics of study participantsa
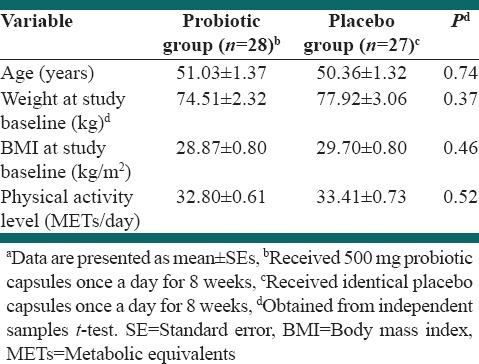
Table 2.
Total dietary intakes of study groups during the studya
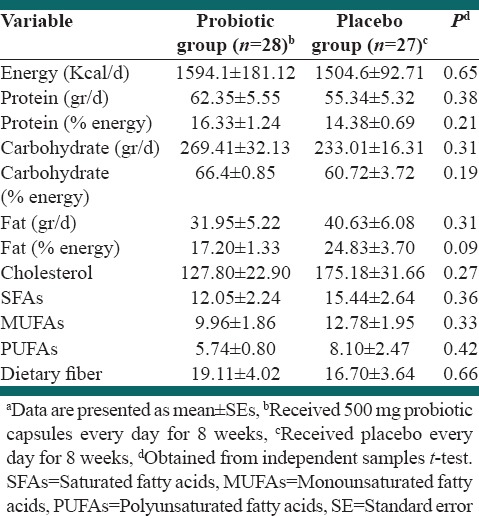
Table 3.
Effects of 8 weeks of probiotic consumption on TC, LDL, HDL, TG/LDL, TG/HDL and HDL/LDL plus SBP and DBP compared with placeboa
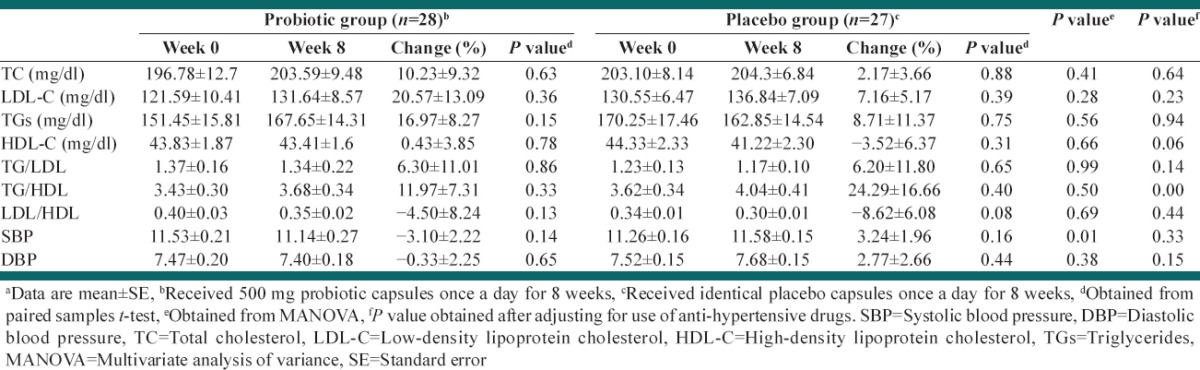
Treatment group led to lower systolic and diastolic blood pressures although the reductions were not statistically significant. On the other hand, systolic and diastolic blood pressures increased slightly in the placebo group, but the changes were not significant. Percent change in systolic blood pressure was significantly different in the probiotic group in comparison with a placebo group (–3.10 ± 2.22 vs. 3.24 ± 1.96), but after adjusting for potential confounders, this significance did not exist anymore.
DISCUSSION
The present study was conducted to evaluate whether probiotic administration, in the form of supplement, can exert effects on TC, LDL-c, HDL-c, TG/LDL, TG/HDL, LDL/HDL as well as systolic and diastolic blood pressures in prediabetic patients.
Our findings show that daily consumption of probiotic capsules did not have significant effects on blood lipid markers including TC, LDL-C, HDL-C as well as TG/LDL-C, TG/HDL-C and LDL-C/HDL-C, after 8 weeks. Changes in HDL-C level and TG/HDL ratio between two groups became significantly different after adjustment for confounders. Mazloom et al.[23] showed that probiotic capsules containing lactic acid bacteria did not have any favorable effects on fasting blood glucose levels, insulin resistance and blood lipids, after 6 weeks of intervention. Other studies show beneficial effects of probiotic consumption in diabetes management: Yadav et al.[13] reported that dahi, a fermented dairy containing lactobacillus bacteria, can delay the onset of glucose intolerance, hyper-insulinemia, dyslipidemia, and oxidative stress in high fructose-fed rats. Ataie-Jafari et al.[24] evaluated the effects of probiotic yogurt in diabetic subjects. Their study showed that daily consumption of 300 g probiotic enriched-yogurt reduced total and LDL cholesterol concentrations compared with the control group. Moroti et al.[25] used symbiotic shake, as a combination of probiotics and prebiotics product, in their intervention group. According to their research, 4 week consumption of symbiotic products can decrease serum TC and TG levels and increase HDL-C concentration, significantly. Lewis and Burmeister[22] conducted a study on 80 hypercholestrolemic volunteers who received two capsules containing lactobacillus acidophilus, 3 times a day for 6 weeks. Similar to our results, they could not show any significant effects of probiotics intake on serum blood lipid. In regard to cholesterol lowering effects of probiotics, several mechanisms can be proposed. Fermentation products of Lactobacillus bacteria, mainly short chain fatty acids, can inhibit enzymatic synthesis of cholesterol, and these bacteria may inhibit absorption of cholesterol by chelating it. They also assimilate and incorporate cholesterol as part of their cell membranes.[25,26] It has been demonstrated that some species of Lactobacillus, Bifidobacterium and Streptococcus bacteria are able to lower cholesterol levels.[27] In the present study, we used capsules containing lactobacillus bacteria and Bifidobacteria as well as other strains and ingredients that did not beneficially affect different markers of blood lipids.
Life-threatening DM complications on one hand and its increasing prevalence, on the other hand, calls for natural and safe strategies to control and delay these outcomes.[22] Impaired fasting glucose or prediabetes is a strong predictor of diabetes[28,29] and it can put individuals at CVD risk.[28] Recently it has been documented that patients with type 2 DM show an alteration in their gut microbial composition; therefore, the use of probiotics towards modifying gut microflora become a new way of regulating glucose metabolism.[18] Our study aimed to evaluate the effects of probiotic supplementation on blood lipids and lipid ratios as well as blood pressure in persons with pre-DM for 8 weeks, and compare these effects with a placebo group. Probiotic capsules in the present study were able to improve systolic blood pressure compared with the placebo. However, this finding did not exist anymore after adjusting for possible confounders. Results of our study are consistent with results from different animal and human studies demonstrating that probiotic administration contributes to improved systolic blood pressure, diastolic blood pressure or both.[9]
It seems that probiotic administration in forms of dairy or other food items function more properly than probiotic capsules. The reason for this fact is that people show more enthusiasm for consuming ordinary foods rather than capsules, especially probiotics with lower familiarity for society. On the other hand, participants might have forgotten to take prescribed supplements regularly although the research executors were continuously in touch with study participants. Moreover, we did not use any biochemical marker to assess individuals’ compliance to prescribed capsules. We did not control other possible probiotic source, especially dairies since they are a part of usual diet and important source for calcium. According to dietary intakes obtained from 24 h. recalls, a probiotic group had lower energy intake from dietary fat that could confound the results although this difference was marginally significant. We noted the point that participants underreported their fat intake since the calculated amounts are below normal levels. Our study has strengths too. Few studies have used purified probiotics in the form of supplements to evaluate their exclusive effects. Numbers of human studies in this area are limited, and most of the trials have done on animals. To the best of our knowledge, the present study was first to evaluate effects of probiotics in prediabetic patients. Our recommendation for future researchers is to control for confounders such as dietary habits, dairy intake and using fermented foods as much as they can and use markers to evaluate patients’ compliance, stool sampling as a detector of bacteria load. Dosage of probiotic used in our study play effective roles on our results. Studies of larger sample size and during longer duration are suggested.
CONCLUSIONS
Our study shows that probiotics do not improve TC, LDL-c, TGs, TG/HDL or LDL/HDL although placebo group had increased TG/HDL and decreased HDL-c that differed significantly from probiotic group. Results of our study show that probiotic administration can lead to improvement in systolic blood pressure. However, these effects did not remain significant after adjustment for confounding variables. There is a need for more powerful experiments in this area with more sample size, higher dosage of probiotics and controlling for possible confounders.
ACKNOWLEDGMENTS
This study was extracted from MSc dissertation, approved by Food Security and Research Center and School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (code 392157). Authors thank staff members of Endocrine and Metabolism Research Center and participants for their cooperation during the study.
Footnotes
Source of Support: Vice Chancellor for Research, Isfahan University of Medical Sciences, founds this study
Conflict of Interest: None declared.
REFERENCES
- 1.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–6. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 2.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–5. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 3.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 4.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for diabetes development. Lancet. 2012;379:2279–90. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee SY, Woo JT. The prediabetic period: Review of clinical aspects. Diabetes Metab J. 2011;35:107–16. doi: 10.4093/dmj.2011.35.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham TM, Fox CS. Implications of rising prediabetes prevalence. Diabetes Care. 2013;36:2139–41. doi: 10.2337/dc13-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao W, Gong W, Wu N, Li Y, Ye K, Lu B, et al. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. 2014;13:37. doi: 10.1186/1476-511X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC, Espinoza-Camacho MA, Jiménez-Morales M, Piña E, et al. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res. 2013;54:2795–9. doi: 10.1194/jlr.M040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal J, Swami G, Kumar M. Probiotics and their effects on metabolic diseases: An update. J Clin Diagn Res. 2013;7:173–7. doi: 10.7860/JCDR/2012/5004.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 11.Association AD. Position statement: Standards of medical care in diabetes-2010. Diabetes Care. 2010;33:S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–93. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 13.Yadav H, Jain S, Sinha P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–8. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75:189–95. [Google Scholar]
- 15.Andersson U, Bränning C, Ahrné S, Molin G, Alenfall J, Onning G, et al. Probiotics lower plasma glucose in the high-fat fed C57BL/6J mouse. Benef Microbes. 2010;1:189–96. doi: 10.3920/BM2009.0036. [DOI] [PubMed] [Google Scholar]
- 16.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–43. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 17.An HM, Park SY, Lee do K, Kim JR, Cha MK, Lee SW, et al. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011;10:116. doi: 10.1186/1476-511X-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejtahed H, Nia J, Rad A, Niafar M, Jafarabadi M, Mofid V. The effects of probiotic yoghurt consumption on blood pressure and serum lipids in type 2 diabetic patients: Randomized clinical trial. Iran J Nutr Sci Food Technol. 2012;6:Pe1–12. [Google Scholar]
- 19.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–9. [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SJ, Burmeister S. A double-blind placebo-controlled study of the effects of Lactobacillus acidophilus on plasma lipids. Eur J Clin Nutr. 2005;59:776–80. doi: 10.1038/sj.ejcn.1602139. [DOI] [PubMed] [Google Scholar]
- 23.Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran J Med Sci. 2013;38:38–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Ataie-Jafari A, Larijani B, Alavi Majd H, Tahbaz F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann Nutr Metab. 2009;54:22–7. doi: 10.1159/000203284. [DOI] [PubMed] [Google Scholar]
- 25.Moroti C, Souza Magri LF, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;22(11):29. doi: 10.1186/1476-511X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabian E, Elmadfa I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann Nutr Metab. 2006;50:387–93. doi: 10.1159/000094304. [DOI] [PubMed] [Google Scholar]
- 27.Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–55. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 29.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care. 2006;29:1872–7. doi: 10.2337/dc05-2141. [DOI] [PubMed] [Google Scholar]


