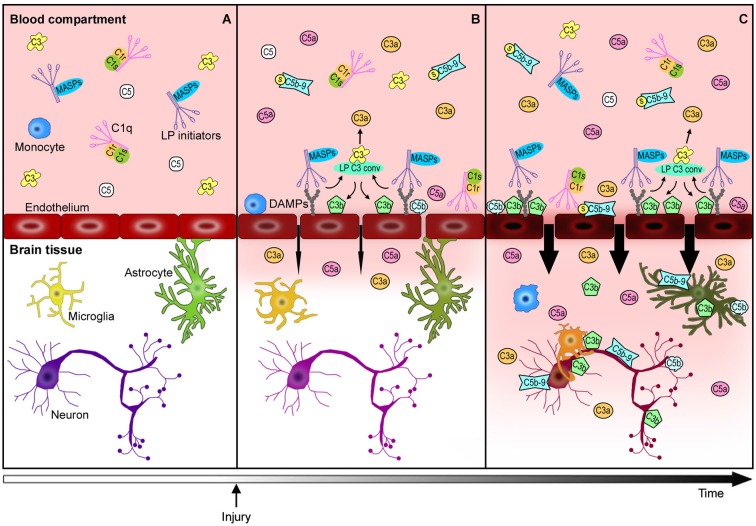
Figure 2.
Complement-mediated endothelial damage: working hypothesis. Healthy brain endothelial cells physically isolate the brain parenchyma from the blood compartment. Under physiological conditions, complement proteins, such as C1q, LP initiators, C3 and C5, monitor the blood and the cell surfaces for potential threats (A). The additional complement factors that normally circulate in the bloodstream, have been omitted in this figure for purposes of clarity and simplification. When a cerebrovascular injury occurs (B), endothelial cells may change their glycosylation profile, leading to the exposure of high density arrays of sugar (DAMPs). LP initiators, through their carbohydrate recognition domains, can recognize and bind altered endothelial cells, leading to LP complement activation (B). Complement activated fragments trigger the expression of adhesion molecules and the release of chemokines and cytokines (not shown) on endothelial cells, resulting in the worsening of the BBB leakage (B→C). The injured brain parenchyma is then rapidly invaded by the full immune arsenal, including the complement proteins, cytokines and immune cells (e.g., monocytes) belonging to the blood compartment, leading to amplification of local damage (C). The overactivation of the complement system in brain tissue leads to: (1) a potent inflammatory response and the recruitment of peripheral immune cells mediated by C3a and C5a anaphylatoxins, (2) direct lysis of neurons and other brain cells, including those that are potentially savable, by C5b-9, (3) opsonization with subsequent microglia/macrophage phagocytosis of the target cells by C3b and C5b. This schema proposes a key role for endothelial cells in triggering local LP complement activation and suggests that targeting the peripheral compartment may represent effective strategy for brain protection from injury and different acute CNS diseases.