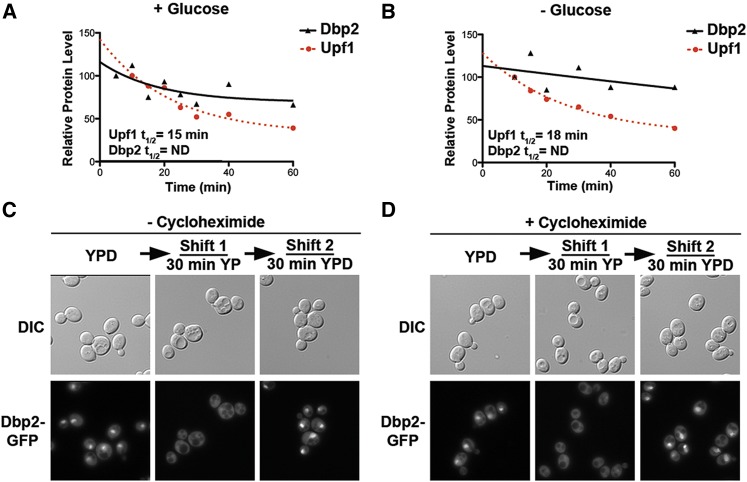
Figure 2.
The change in cellular localization of Dbp2 is due to nuclear transport, not protein turnover. (A and B) Dbp2 protein exhibits similar stability irrespective of the presence of glucose in the media. The stability of Dbp2 protein in the presence of glucose (A) or following glucose deprivation (B) by adding cycloheximide, as previously described to prevent new protein synthesis (Castoralova et al. 2012). Samples were removed at 5-, 10-, 20-, 30-, 40-, and 60-min increments and subjected to Western blotting with rabbit polyclonal anti-Dbp2. Dbp2 levels were quantified with respect to Pgk1 and are presented graphically. Upf1, another RNA helicase, has a reported half-life of ∼16 min (Ruiz-Echevarria et al. 1998) and is included as a control for efficient translational shutoff. Dbp2 half-lives could not be determined for either growth conditions because they do not decrease substantially within a 1-hr time frame. (C) Readdition of glucose to glucose-deprived cells restores nuclear Dbp2 signal. Dbp2–GFP-expressing cells were subjected to a 30-min glucose deprivation, to ensure complete cytosolic redistribution, and were then resuspended in fresh media with saturating glucose (2%). Dbp2–GFP was visualized before glucose removal (0 min), following deprivation, and after 30 min incubation with fresh, glucose-containing media. Dbp2 localization was visualized by fluorescent microscopy as above. Note that Dbp2–GFP-expressing strains show reduced signal in the absence of glucose (middle). However, this is not due to a change in Dbp2 protein levels (see Figure 2A). (D) New protein synthesis is not necessary for restoration of nuclear signal upon glucose readdition. Dbp2–GFP localization was determined as in C, but in the presence of cyclohexamide to block translation.