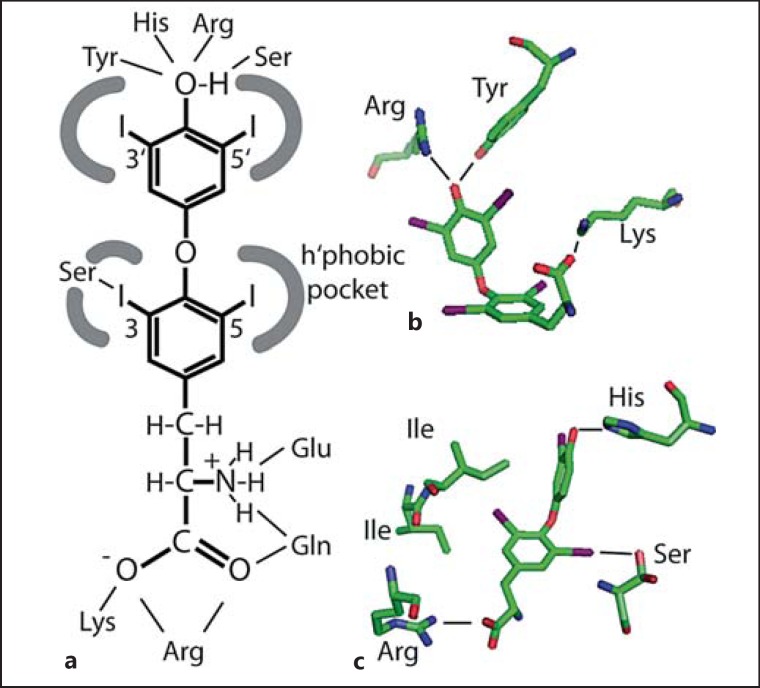
Fig. 4.
Schematic representation how TH interact with proteins. a The iodothyronine molecule is largely hydrophobic, but the 4′- and aminopropionic acid moieties are hydrophilic. Hydrophobic interactions account for most of the TH binding, while interactions with the 4′-hydroxyl and sometimes the aminopropionic acid moiety confer additional specificity. b Polar contacts in the binding pocket for T4 in HSA. The hormone is in a cisoid conformation, i.e. phenolic ring and aminopropionic acid are on the same side of the tyrosyl ring. c Polar and hydrophobic contacts in the binding pocket for T3 in TR-β. The ligand is in the extended conformation. Note the conserved polar contact of Ser with iodine.