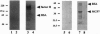Abstract
The pathogenic Gram-positive bacterium Streptococcus pyogenes (group A streptococcus) is the causative agent of numerous suppurative diseases of human skin. The M protein of S. pyogenes mediates the adherence of the bacterium to keratinocytes, the most numerous cell type in the epidermis. In this study, we have constructed and analyzed a series of mutant M proteins and have shown that the C repeat domain of the M molecule is responsible for cell recognition. The binding of factor H, a serum regulator of complement activation, to the C repeat region of M protein blocked bacterial adherence. Factor H is a member of a large family of complement regulatory proteins that share a homologous structural motif termed the short consensus repeat. Membrane cofactor protein (MCP), or CD46, is a short consensus repeat-containing protein found on the surface of keratinocytes, and purified MCP could competitively inhibit the adherence of S. pyogenes to these cells. Furthermore, the M protein was found to bind directly to MCP, whereas mutant M proteins that lacked the C repeat domain did not bind MCP, suggesting that recognition of MCP plays an important role in the ability of the streptococcus to adhere to keratinocytes.
Full text
PDF


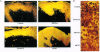
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. M., Brown M. C., Nunge M., Krych M., Atkinson J. P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991 Nov 1;147(9):3005–3011. [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronze M. S., Courtney H. S., Dale J. B. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J Immunol. 1992 Feb 1;148(3):888–893. [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein. Sci Am. 1991 Jun;264(6):58–65. doi: 10.1038/scientificamerican0691-58. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E., Horwitz P. A., Caparon M. G. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992 Dec;60(12):5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Horstmann R. D., Sievertsen H. J., Knobloch J., Fischetti V. A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Abraham S., Caparon M., Falk P., St Geme J. W., 3rd, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993 Jun 4;73(5):887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- Kupper T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990 Jun;94(6 Suppl):146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Liszewski M. K., Post T. W., Atkinson J. P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- Manchester M., Liszewski M. K., Atkinson J. P., Oldstone M. B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Pentland A. P., Falk P., Caparon M. G. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J Clin Invest. 1994 Sep;94(3):965–977. doi: 10.1172/JCI117463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J., Price J. A., Maguin E., Scott J. R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993 May;8(5):809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Retnoningrum D. S., Cleary P. P. M12 protein from Streptococcus pyogenes is a receptor for immunoglobulin G3 and human albumin. Infect Immun. 1994 Jun;62(6):2387–2394. doi: 10.1128/iai.62.6.2387-2394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K., Shiraishi S., Shirakata Y., Kobayashi Y., Seya T., Miki Y. Expression and characterization of membrane co-factor protein (MCP) in human skin. J Invest Dermatol. 1991 Oct;97(4):722–724. doi: 10.1111/1523-1747.ep12484155. [DOI] [PubMed] [Google Scholar]
- Scott J. R. A turbid plaque-forming mutant of phage P1 that cannot lysogenize Escherichia coli. Virology. 1974 Dec;62(2):344–349. doi: 10.1016/0042-6822(74)90397-3. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Guenthner P. C., Malone L. M., Fischetti V. A. Conversion of an M- group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986 Nov 1;164(5):1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Stevens D. L. Invasive group A streptococcus infections. Clin Infect Dis. 1992 Jan;14(1):2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W. Differences between streptococcal infections of the throat and of the skin. I. N Engl J Med. 1970 Jan 1;282(1):23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Law S. K., Levine R. P., Cleary P. P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985 Jan;134(1):500–505. [PubMed] [Google Scholar]