Abstract
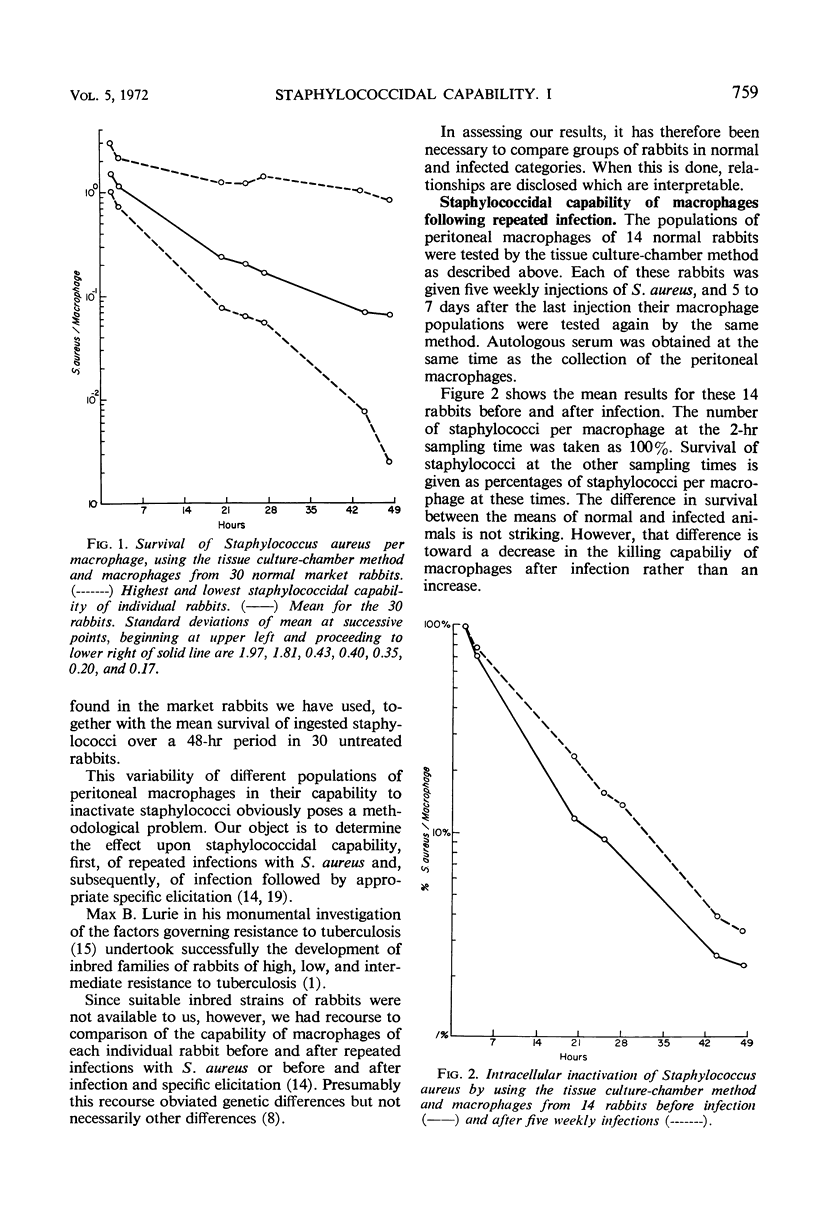
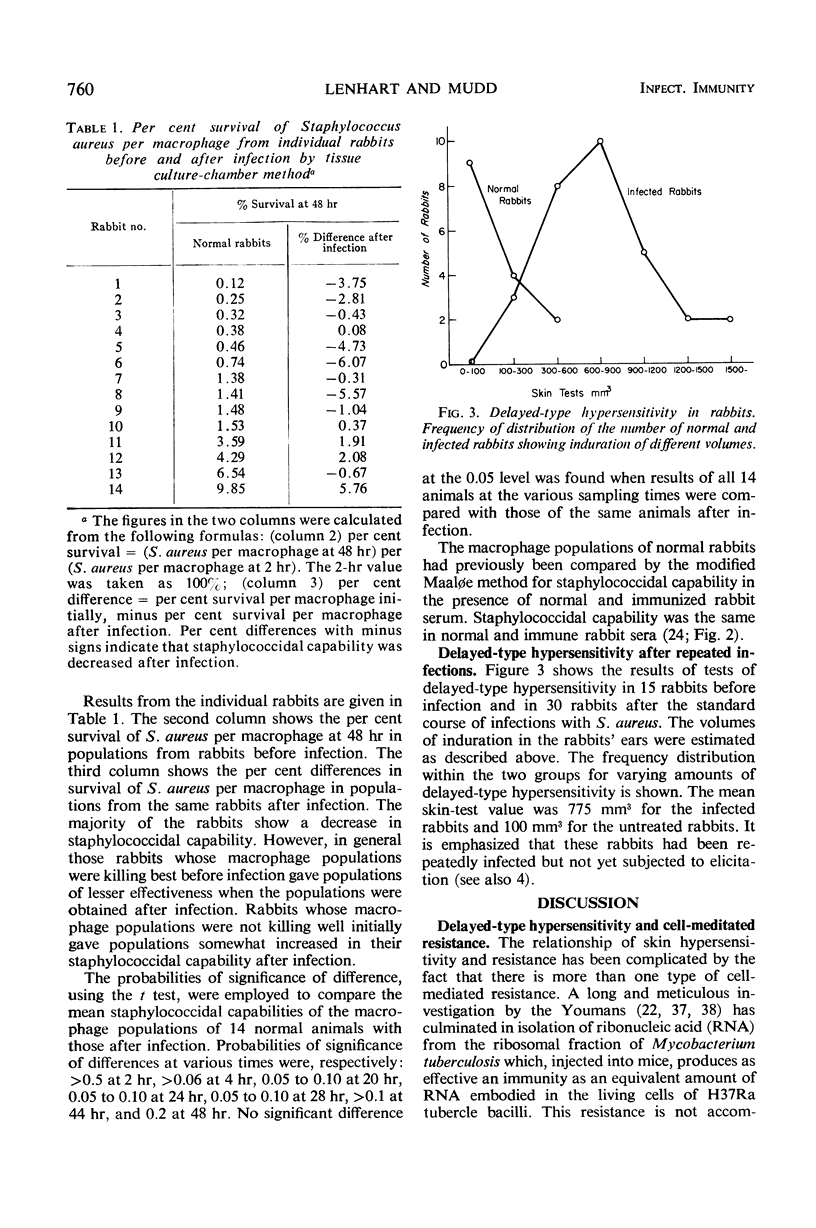
The staphylococcidal capability of populations of peritoneal macrophages in rabbits has been measured before and after repeated infections with Staphylococcus aureus. Such rabbits after infection showed delayed-type hypersensitivity to S. aureus antigens, but the staphylococcidal capability of the peritoneal macrophages was not increased. This result at the cellular level is in agreement with previous assessment in vivo of the consequences of staphylococcal infection. Pathways to cell-mediated resistance, with and without delayed-type hypersensitivity, are presented.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., ZAPPASODI P., LURIE M. B. Metabolic studies on mononuclear cells from rabbits of varying genetic resistance to tuberculosis. I. Studies on cells of normal noninfected animals. Am Rev Respir Dis. 1961 Sep;84:364–370. doi: 10.1164/arrd.1961.84.3.364. [DOI] [PubMed] [Google Scholar]
- BAKER A. G. Staphyloccus bacteriophage lysate: topical and parenteral use in allergic patients. Pa Med J. 1963 Apr;66:25–28. [PubMed] [Google Scholar]
- BAKER A. G. Treatment of chronic bronchial asthma; aerosol of staphylococcus bacteriophage lysate as an adjunct to systemic hyposensitization. Am Pract Dig Treat. 1958 Apr;9(4):591–598. [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Macrophages and delayed-type hypersensitivity. Semin Hematol. 1970 Apr;7(2):215–224. [PubMed] [Google Scholar]
- Cluff L. E. Cellular reactions in the pathogenesis of staphylococcal infections. Ann N Y Acad Sci. 1965 Jul 23;128(1):214–230. doi: 10.1111/j.1749-6632.1965.tb11640.x. [DOI] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G. Structure of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):109–124. [PubMed] [Google Scholar]
- JOHANOVSKY J. Role of hypersensitivity in experimental staphylococcal infection. Nature. 1958 Nov 22;182(4647):1454–1454. doi: 10.1038/1821454a0. [DOI] [PubMed] [Google Scholar]
- KAPRAL F. A., SHAYEGANI M. G. Intracellular survival of staphylococci. J Exp Med. 1959 Jul 1;110(1):123–138. doi: 10.1084/jem.110.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J. J., Berman D. T. Immunobiological activity of cell wall antigens of Staphylococcus aureus. Infect Immun. 1971 Sep;4(3):205–211. doi: 10.1128/iai.4.3.205-211.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart N., Mudd S. Staphylococcidal capability of rabbit peritoneal macrophages in relation to infection and elicitation: induction and elicitation of activated macrophages. Infect Immun. 1972 May;5(5):763–768. doi: 10.1128/iai.5.5.763-768.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Mudd S. Resistance against Staphylococcus aureus. JAMA. 1971 Dec 13;218(11):1671–1673. doi: 10.1001/jama.218.11.1671. [DOI] [PubMed] [Google Scholar]
- Mudd S., Taubler J. H., Baker A. G. Delayed-type hypersensitivity to Staphylococcus aureus in human subjects. J Reticuloendothel Soc. 1970 Nov;8(5):493–498. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMON G. G., Jr, SYMONDS M. STAPHAGE LYSATE THERAPY IN CHRONIC STAPHYLOCOCCAL INFECTIONS. J Med Soc N J. 1963 May;60:188–193. [PubMed] [Google Scholar]
- SHAYEGANI M. G., KAPRAL F. A., MUDD S. PHAGOCYTOSIS AND INTRACELLULAR KILLING OF STAPHYLOCOCCUS AUREUS BY HUMAN AND RABBIT BLOOD LEUKOCYTES. J Immunol. 1964 Jul;93:88–93. [PubMed] [Google Scholar]
- SHAYEGANI M. G., KAPRAL F. A. The eventual intracellular destruction of staphylococci by mononuclear cells. J Gen Microbiol. 1962 Dec;29:637–644. doi: 10.1099/00221287-29-4-637. [DOI] [PubMed] [Google Scholar]
- SHAYEGANI M. G., KAPRAL F. A. The immediate fate of staphylococci after phagocytosis. J Gen Microbiol. 1962 Dec;29:625–636. doi: 10.1099/00221287-29-4-625. [DOI] [PubMed] [Google Scholar]
- Shayegani M. Failure of Immune Sera to Enhance Significantly Phagocytosis of Staphylococus aureus: Nonspecific Adsorption of Phagocytosis-Promoting Factors. Infect Immun. 1970 Dec;2(6):742–749. doi: 10.1128/iai.2.6.742-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. T. Autogenous vaccines in theory and in practice. Personal experience. Arch Intern Med. 1970 Feb;125(2):344–350. [PubMed] [Google Scholar]
- Taubler J. H., Grieb M., Mudd S. Immunologically Induced and Elicited Local Resistance to Staphylococcus aureus. Infect Immun. 1970 Dec;2(6):757–761. doi: 10.1128/iai.2.6.757-761.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Andersson B. Isolation of lymphoid cells with active surface receptor sites. Annu Rev Microbiol. 1971;25:291–308. doi: 10.1146/annurev.mi.25.100171.001451. [DOI] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]


