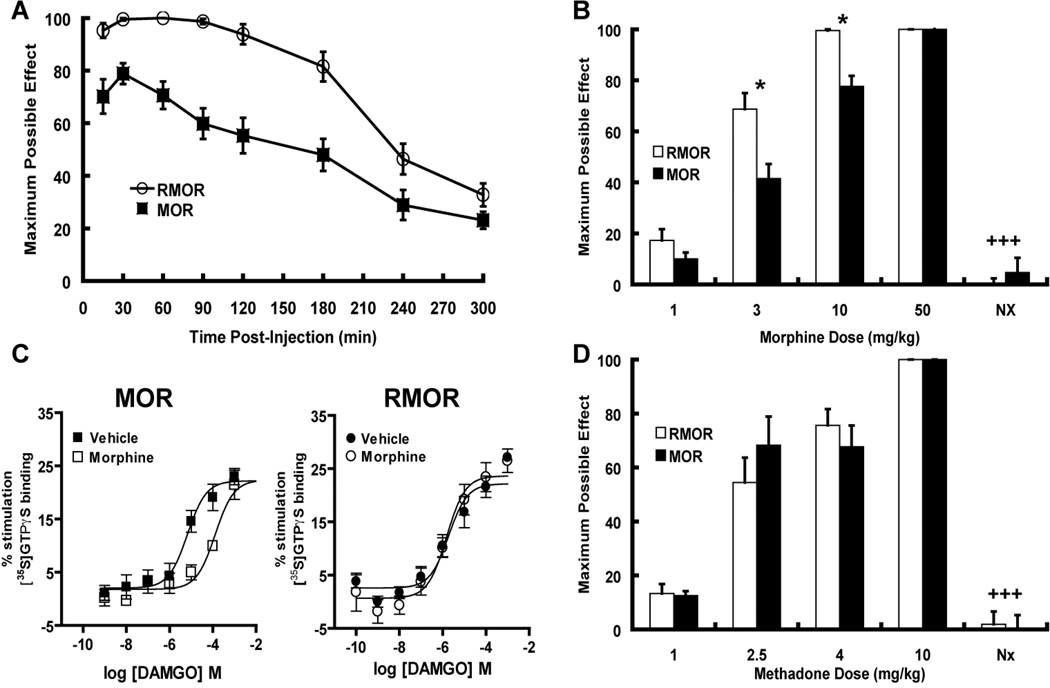
Figure 3. Antinociception in WT MOR and RMOR knock-in mice.
A. Enhanced and prolonged morphine-induced antinociception in RMOR knock-in mice. Antinociceptive responses were measured with the hot-plate response latency test (56°C) after morphine treatment (10 mg/kg, sc). A response endpoint was defined as latency to either lick the fore- or hindpaws or flick the hindpaws. To avoid tissue damage, mice were exposed to the hot-plate for a maximum of 20 seconds. Data are reported as the mean ± SEM of percent maximum possible effect (MPE) using the following formula: 100% x [(drug response time – basal response time)/(20 s – basal response time)]. A two-way analysis of variance revealed that the MPE curve for RMOR mice (n=17) mice was significantly greater and prolonged relative to the MOR mice (n=17) as indicated by a significant genotype [p<.001, F(1,7)=28.05] and genotype X time interaction effect [p<.001, F(1,7)=4.97]. B. Dose-dependent morphine-antinociception. Antinociceptive responses were determined with the hot-plate test and data are reported as mean ± SEM of MPE (see A). Separate groups of mice for both genotypes (n=7–9) were injected with the doses of morphine indicated and assessed for antinociception 30 min later. To test whether the antinociceptive responses were mediated by opioid receptors, a final grouping was injected with morphine (10 mg/kg) followed by naloxone (2 mg/kg). RMOR knock-in mice showed enhanced antinociception at 3 and 10 mg/kg doses (RMOR vs. WT MOR scores for MPE at respective morphine doses, student’s t-test, *p<.03) with the latter dose inducing the maximum possible response (100%) in the mutant mice. At the highest dose tested (50 mg/kg) both genotypes exhibited the maximum possible response (100%). For both genotypes, antinociception induced by 10 mg/kg of morphine was reversed by treatment with 2 mg/kg of the opioid antagonist naloxone (morphine 10 mg/kg with and without naloxone 2 mg/kg treatment for each genotype respectively, student’s t-test +++p<.001). C. MOR desensitization in the brainstem following acute morphine treatment. Agonist-mediated [35S]GTPγS binding was measured in brainstem membranes of WT MOR and RMOR knock-in mice with increasing concentrations of DAMGO. Left Panel. Binding in WT MOR mice was significantly reduced (p<0.01) following acute morphine-treatment (10 mg/kg s.c. 30 min; EC50 = 428 ± 141 µM; open squares ) compared to vehicle-treated mice (EC50 = 2.35 ± 0.9 µM; closed squares). Right Panel. Binding in RMOR knock-in mice was not significantly changed (p>0.05) following acute morphine-treatment (EC50 = 3.29 ± 1.4 µM; open circles) compared to vehicle-treated mice (EC50 = 1.16 ± 0.6 µM; closed circles). Data were analyzed by nonlinear regression using GraphPad Prism software and are presented as means ± SEM of at least three experiments performed in triplicate with an investigator blind to genotype. D. Enhanced antinociception in RMOR knock-in mice is morphine-specific. Separate groups of mice for both genotypes (n=8–10) were injected with the doses of methadone indicated (1–10 mg/kg,) and assessed for antinociception. Methadone induced a dose-dependent increase in antinociceptive response with no genotypic differences. For both genotypes, antinociception induced by 4 mg/kg of methadone was reversed by treatment with 2 mg/kg of the opioid antagonist naloxone (methadone 4 mg/kg with and without naloxone 2 mg/kg treatment for each genotype respectively, student’s t-test +++p<.001). Thus, enhanced opioid-induced antinociception observed in the RMOR knock-in mice is agonist-specific, and naloxone-reversible. Reproduced with permission from Kim, J.A., Bartlett, S., He, L., Nielsen, C.K., Chang, A.M., Kharazia, V., Waldhoer, M., Ou, C.J., Taylor, S., Ferwerda, M., Cado, D., Whistler, J.L., 2008. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr. Biol. 18, 129–135.