Abstract
Since obligate avian brood parasites depend completely on the effort of other host species for rearing their progeny, the availability of hosts will be a critical resource for their life history. Circumstantial evidence suggests that intense competition for host species may exist not only within but also between species. So far, however, few studies have demonstrated whether the interspecific competition really occurs in the system of avian brood parasitism and how the nature of brood parasitism is related to their niche evolution. Using the occurrence data of five avian brood parasites from two sources of nationwide bird surveys in South Korea and publically available environmental/climatic data, we identified their distribution patterns and ecological niches, and applied species distribution modeling to infer the effect of interspecific competition on their spatial distribution. We found that the distribution patterns of five avian brood parasites could be characterized by altitude and climatic conditions, but overall their spatial ranges and ecological niches extensively overlapped with each other. We also found that the predicted distribution areas of each species were generally comparable to the realized distribution areas, and the numbers of individuals in areas where multiple species were predicted to coexist showed positive relationships among species. In conclusion, despite following different coevolutionary trajectories to adapt to their respect host species, five species of avian brood parasites breeding in South Korea occupied broadly similar ecological niches, implying that they tend to conserve ancestral preferences for ecological conditions. Furthermore, our results indicated that contrary to expectation interspecific competition for host availability between avian brood parasites seemed to be trivial, and thus, play little role in shaping their spatial distributions and ecological niches. Future studies, including the complete ranges of avian brood parasites and ecological niches of host species, will be worthwhile to further elucidate these issues.
Keywords: Avian brood parasitism, Cuculus, ecological niche, interspecific competition, spatial distribution, species distribution modeling
Introduction
Interspecific brood parasitism is a breeding strategy in which brood parasites lay their eggs in the nests of other species, called hosts, and shift parental duties onto them (Wyllie 1981; Davies 2000; Payne 2005). In birds, about 100 species (ca. 1% of all known bird species) are known to be obligate brood parasites; that is, they never build nests or provide food for their progeny (Davies 2000; Payne 2005). Because of the detrimental effect of brood parasitism, hosts often develop defensive strategies, such as parasitic egg discrimination and rejection, which leads to counter-adaptations such as egg mimicry by brood parasites (Brooke and Davies 1988; Davies and Brooke 1989; Stokke et al. 2002; Avilés et al. 2006; Kilner 2006). In addition, some host species develop further strategies such as egg color polymorphism, to defeat egg mimicry by brood parasites (Lee and Yoo 2004; Takasu 2005; Yang et al. 2010; Liang et al. 2012). These interesting aspects of avian brood parasitism have drawn much research interest, especially in the area of evolutionary biology as a model system of coevolution. Because of this, we have now accumulated much knowledge revealing their hidden life history (Rothstein 1990; Rothstein and Robinson 1998; Davies 2000).
Parasitic relationships, including brood parasitism, may also provide an excellent research subject in the field of macroecology for studying distribution patterns, ecological niches, and species interactions (Ricklefs 2010; Wisz et al. 2013), and this knowledge may in turn provide useful insights into not only evolutionary questions but also conservation issue. Currently, however, we have a relatively narrow range of information about this aspect of avian brood parasitism. As the availability of host species is a critical resource in the life history of avian brood parasites, the patterns of their distribution and abundance should be shaped not only by their own ecological needs related to climate and food availability but also by those of their host species (Davies 2000; Ducatez 2014). In particular, some avian brood parasites such as Cuculus species are known to have host specificity; in other words, each species or each individual within a species parasitizes a specific host species rather than exploiting multiple species, indicating that they may adopt different adaptive trajectories according to the host species on which they specialize (Marchetti et al. 1998; Gibbs et al. 2000; Nakamura et al. 2005; Davies et al. 2006; Madden and Davies 2006; Fossøy et al. 2011). This host specificity may provide a good opportunity to clarify fully the effect of biotic factors in the study of species distribution. Brood parasites may either adjust their range and ecological niche to those of any suitable hosts or confine their available host species to those in their current range and ecological niche. Such a tendency may vary among species or among individuals within a species, being a potential driving force of niche differentiation and speciation.
Different species of avian brood parasites may interact antagonistically to secure access to host species (Davies 2000). Competing species may end up separating their ranges, limiting their preference to a specific host species, or existing sympatrically but differentiating their ecological niches according to their host species. Furthermore, this competition over host species may underlie speciation in avian brood parasites (Davies 2000). Much circumstantial evidence supports the probability that such competition does occur in nature. For example, some host species in Japan and China are known to be parasitized as secondary hosts by multiple Cuculus species (Nakamura et al. 1998; Yang et al. 2012), implying the presence of potential competition between different brood parasites. Similar incidents were observed in Korea, where Phoenicurus auroreus seems to be used by two Cuculus species: C. canorus and C. optatus (J.-W. Lee, personal observation). Furthermore, when the competition between two species is released due to allopatric distributions, a species of brood parasites often exploits a typical host used exclusively by the other species where their ranges overlap, being an example of character release (Higuchi and Sato 1984). However, whether these incidences happened directly as a result of competition between species is still unclear, because the patterns of species distribution and interaction may also be influenced by many other factors such as abiotic environmental conditions (Dunson and Travis 1991; Lloyd and Palmer 1998; Martin 2001; Sexton et al. 2009). Further direct evidence of the presence of such competition and its outcome is still needed to clarify this issue.
In this study, we compared the spatial distribution patterns and ecological niches of five avian brood parasites breeding in Korea: Cuculus canorus, C. optatus, C. micropterus, C. poliocephalus, and Hierococcyx hyperythrus. They are known to not be globally threatened, although some of their local populations are reported to be declining (Birdlife International 2014). C. canorus, which is one of most studied avian brood parasites, has an extensive breeding range, occurring across Eurasia, and C. optatus is distributed from European Russia through Siberia and East Asia to the Pacific Coast of Eurasia during the breeding season (Birdlife International 2014). The three other species have much smaller breeding ranges, mainly restricted to Northeast Asia (Birdlife International 2014). In Korea, all five species are summer visitors (Lee et al. 2000) but information as to which species are regularly parasitized by each of the brood parasites is relatively limited because there are few relevant published studies. Nevertheless, some published as well as unpublished data are available, from which host species might be able to be inferred. C. canorus mainly use Paradoxornis webbianus in mainland Korea and Emberiza cioides in Jeju Island (Lee et al. 2000; Lee and Yoo 2004; Kang et al. 2009). Besides these species, Phoenicurus auroeus, Saxicola torquatus, and Motacilla cinerea are also observed to be occasionally parasitized by C. canorus. C. optatus seems to usually exploit Phylloscopus coronatus but occasionally Phoenicurus auroeus. In Jeju Island, it is also reported that Terpsiphone atrocaudata is parasitized by C. optatus (Kim 2011). The primary host of C. poliocephalus is known to be Cettia diphone and potentially Troglodytes troglodytes (Kang et al. 2009). Unfortunately, it is still unclear which species is regularly parasitized by C. micropterus in Korea. For H. hyperythrus, anecdotal observations, such as photographs of eggs or nestlings taken by local birdwatchers, suggest Cyanoptila cyanomelana as a potential primary host. Overall, each species of brood parasite is likely to exploit different primary host species, but, as mentioned earlier, it is also likely that some host species such as Phoenicurus auroeus, will be parasitized by multiple species of brood parasites.
The specific aims of this study are to identify the spatial and ecological niches of five sympatric brood parasites breeding in Korea and, based on this, to infer whether they actually compete with each other over spatial use and host availability. To achieve this, we first investigated their distribution patterns and relative abundances, and their ecological correlates. Secondly, we compared the ecological niches of each species estimated based on the climate conditions and altitude of the region where they were observed. Finally, species distribution modeling (SDM) using Maxent (Phillips et al. 2006) was carried out to determine ecologically important factors shaping their distribution patterns. The results from SDMs can also be applied to infer the presence of species competition by comparing predicted ranges with the realized distribution (Leathwick and Austin 2001; Guisan and Thuiller 2005; Elith and Leathwick 2009; Engler et al. 2013). Adopting this approach, we inferred the relative effect of ecological factors and species competition on the pattern of their spatial distribution.
Materials and Methods
Study area
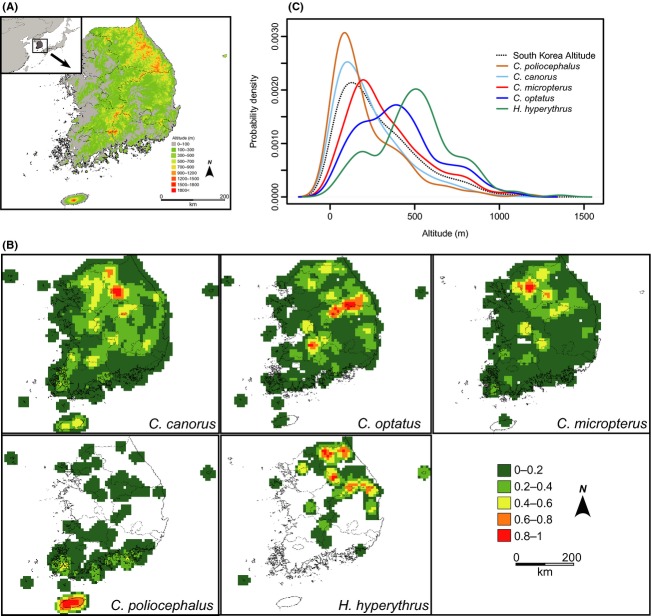
The study area covers the mainland of South Korea and major islands, including the largest island, Jeju-do, which is located at the south of the Korean peninsula (Fig. 1A). South Korea is located in Northeast Asia and its approximate coordinates are 36°N, 128°E. About 70% of the mainland of South Korea consists of uplands or mountains, most of which are in mountain ranges along the eastern part of the mainland (Fig. 1A). The rest consists of lowlands lying along the west coast and major rivers. The highest elevation can be found at Jeju-do (1950 m). The climate of South Korea belongs to a temperate zone with four distinct seasons. Summers are humid and hot while winters are usually dry and cold. The weather in spring and autumn is mild but their durations are short.
Figure 1.
(A) The topographic map of South Korea and its largest island, Jeju-do. (B) Distribution map of the five species of avian brood parasites and the density of observed individuals analyzed with a 5-min cell size and circular neighborhood of 0.4 degrees. Note that the legends indicating the number of observed individuals are standardized for brevity and the maximum number of individuals represented by one vary among the species: 152 individuals for C. canorus, 52 for C. optatus, 111 for C. micropterus, 150 for C. poliocephalus, and 21 for H. hyperythrus. (C) Kernel density plots of the occurrence of the five avian brood parasites according to the altitude of South Korea.
Study species and occurrence data
The georeferenced occurrence data of five avian brood parasites breeding in Korea were obtained from two sources of surveys: the third nationwide environmental study by the National Institute of Environmental Research and natural resource studies by the Korea National Park Research Institute. The former study was carried out from 2006 to 2012 across the whole of South Korea (ca. 99,000 km2), except for national parks (ca. 6581 km2), covering virtually all areas of South Korea. This survey was carried out based on 824 maps that cover South Korea at a 1:25,000 scale. Each map was divided into nine sections (3 × 3) approximately 4 × 5 km in size. Each year, 90–125 maps were surveyed during the survey period by over 70 trained ornithologists and birdwatchers. An actual survey was conducted at least once in each season in five sections near the center, excluding the four sections located at the four corners of the map. There were eight survey days per map. Both line transects and point counts were used as census methods, and the location and the number of birds seen or heard during the survey were recorded. The latter study, which covers the 20 national parks in Korea every year, was carried out by approximately 60 trained ornithologists and birdwatchers applying the same methods as the former survey. For each national park, we used 1 year of survey results chosen randomly from the data collected between 2006 and 2011 in order to be comparable to the former study in terms of the number of surveys and survey periods. The total number of survey days per year per national park ranged from 10 to 30, depending on the size of the park, but overall, similar survey efforts made. Combined, the studies of birds covered virtually all regions of mainland South Korea and major islands with similar amounts of survey effort. Furthermore, the males of each species produce loud, clear, and distinctive calls throughout their breeding seasons, which allowed us to easily spot the birds, even from a distance. Therefore, the quality of the occurrence data was unlikely to be biased in terms of the sampling efforts and survey area. We used the occurrence data collected between April and August when those avian brood parasites breed in Korea as this study was focused on breeding birds.
Spatial and environmental analyses
The occurrence data were first plotted in DIVA-GIS 7.5 (Hijmans et al. 2012) to visually verify data precision, and then the species richness and distribution patterns of each species were analyzed based on point to grid analysis with a raster cell size of 0.0833 (ca. 7 km) and a circular neighborhood option of 0.4 degrees (ca. 35 km). Throughout the study, we used the same cell size and circular neighborhood option as above when we extracted the number of individuals recorded in each cell for density analysis. Altitude and land cover data were extracted from the SRTM 30 (available at: http://srtm.csi.cgiar.org) and GLC 2000 (available at: http://bioval.jrc.ec.europa.eu/products/glc2000/products.php) databases, respectively. Current climatic data within the study area were obtained from the most commonly referenced database, Worldclim v. 1.4 (Hijmans et al. 2005), with a resolution of 2.5 arc-min. Nineteen bioclimatic variables used in the Worldclim database are derived from monthly temperature and precipitation values, representing averages from 1950 to 2000 (Hijmans et al. 2005). Because some of those bioclimatic variables are highly intercorrelated, we carried out a principal component analysis (PCA) to generate an uncorrelated dataset, and finally, we adopted the first three principal components (PCs) with eigenvalues >1 after varimax rotation by the Kaiser criterion for the analysis of ecological niches. Visual comparison of the ecological niche and altitude that each species took up was carried out using kernel probability density plots in R version 3.0.2 (R Core Team 2013), and a Pianka index was calculated using ECOSIM 7.0 (Entsminger 2012) to quantify the degree of niche overlap between species. Values of this index close to 1.0 indicate a complete overlap of niches between species, while values close to 0 represent clear niche separation between species. The statistical significance of the observed overlap was tested using the randomization algorithm built into ECOSIM 7.0 (Entsminger 2012).
Species distribution modeling
To predict the area of potential distribution, we carried out species distribution modeling using Maxent vers. 3.3.3k (Phillips et al. 2006). The 19 bioclimatic variables, altitude, and the type of land cover were included as environmental variables in the model. Despite potential over-parameterization due to high correlations between variables, we included all bioclimatic variables in order to explore all possibilities. This could be allowed in Maxent, because the program has a smoothing procedure called regularization, which relaxes the need to only choose uncorrelated environment variables (Phillips et al. 2006; Hastie et al. 2009; Elith et al. 2011). A randomly selected 75% of the presence records were used for model training and the remaining 25% for test points. We set subsample for the run-type option and 5000 for the maximum number of iterations of the optimization algorithm. For other options, we applied the default settings. The procedures were replicated 15 times, from which we obtained average values. For model evaluation, we provided the score of the area under the receiver operating characteristic curve (AUC). Although the AUC is one of the most widely used parameters to evaluate model performance, it is highly sensitive to model conditions such as the size of samples and backgrounds, especially in a model using presence-only data, such as Maxent (Townsend Peterson et al. 2007; Lobo et al. 2008; Phillips and Dudík 2008; Phillips et al. 2009; Jiménez-Valverde 2012). However, it is also true that there is no promising alternative to this for model evaluation. Therefore, we provided not only AUC scores but also training and test omission rates at 10% training presence (Jiménez-Valverde et al. 2008). The main environmental factors that considerably contributed to the Maxent model were determined by a jackknife test using AUC on test data. The logistic output of the model prediction for habitat suitability was projected in DIVA-GIS 7.0 (Hijmans et al. 2012), in which 0 represents non-suitable and 1 indicates fully suitable. We also generated a binary raster of potential distribution (presence/absence) using the logistic threshold of ten percentile training presence, from which we derived the predicted area of the sympatric presence of multiple species.
Results
Distribution patterns and species interactions
The number of data points used in the spatial analysis varied among species: 3462 for C. canorus, 1533 for C. micropterus, 858 for C. optatus, 526 for C. poliocephalus, and 191 for H. hyperythrus. These differences may reflect the relative abundances of the five species in Korea. They also showed various distribution patterns according to species. C. canorus was the most common, observed in almost all regions of South Korea, but with a higher density in the north (Fig. 1B). C. micropterus showed a similar distribution pattern to C. canorus. C. optatus and H. hyperythrus were less common than the former two species, mainly occurring along the mountain range, but the distribution of H. hyperythrus was much more restricted to higher elevations. The density of C. poliocephalus was highest in Jeju Island, followed by the southern part of mainland Korea. In mainland regions, they seemed to be much rarer and patchily distributed (Fig. 1B). Overall, the species were likely to have different altitudinal preferences (Fig. 1C). The lowest median elevation was found in C. poliocephalus (141 m; 1st–3rd quartiles: 67–319 m), and the highest was in H. hyperythrus (487 m, 1st–3rd quartiles: 369–602 m). In between them, the median elevations for C. canorus, C. micropterus, and C. optatus were 184 m (1st–3rd quartiles: 86–344 m), 271 m (1st–3rd quartiles: 176–455 m), and 381 m (1st–3rd quartiles: 369–602 m), respectively. Considering the frequency distribution of altitudes in South Korea, C. canorus and C. poliocephalus seemed to prefer lower altitudes while C. micropterus, C. optatus, and H. hyperythrus appeared to be observed more frequently at higher altitudes (Fig. 1C). The degree of altitudinal overlap estimated by the Pianka index was largest between C. poliocephalus and C. canorus (0.98) and smallest between H. hyperythrus and C. poliocephalus (0.48).
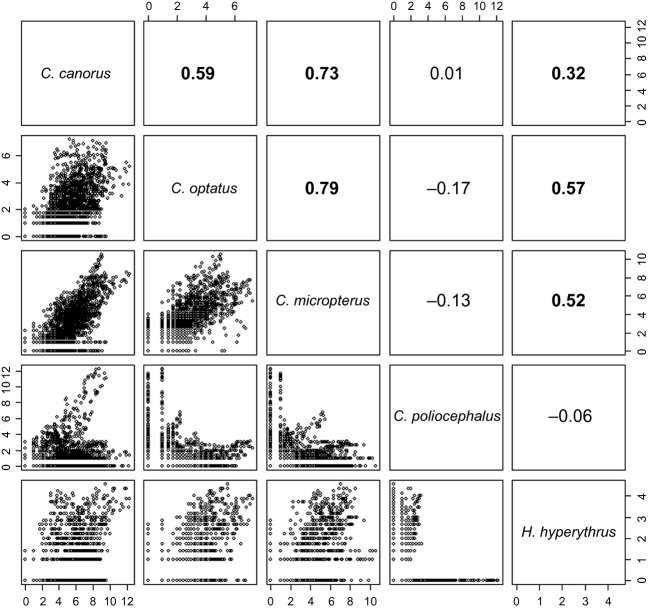
The number of observed individuals of species occurring sympatrically appeared to be positively correlated with each other (Fig. 2). Among those, C. optatus and C. micropterus showed the strongest correlation (rs = 0.79), and the next was between C. canorus and C. micropterus (rs = 0.73). C. canorus and C. optatus also showed a positive correlation in abundance, but the strength was weaker (rs = 0.59). Due to geographic segregation, the abundance of C. poliocephalus was not related to those of other species, but in the places where the abundance of C. poliocephalus was high (>10; e.g., Jeju island), its frequency of occurrence was positively correlated with that of C. canorus (rs = 0.70). The abundance of H. hyperythrus was also positively correlated with those of C. canorus, C. optatus, and C. micropterus, but the relationship was moderate (Fig. 2).
Figure 2.
The pairwise comparison of the relative occurrence between the species. The dots in the lower diagonal represent the square-rooted number of individuals observed in 5-min cells with circular neighborhood of 0.4 degree. The numbers in the upper diagonal represent the spearman rank correlation coefficients of the counterparts. Statistically significant relationships are in bold.
Realized ecological niche and its overlap
To test for differences in ecological niches between these five species, principal component analysis (PCA) was applied. The first three PCs with eigenvalues larger than one explained 91.6% of the total variance of the data (Table 1). PC1 best explained the variability of winter temperature and the seasonality of weather including temperature and precipitation. The winter temperature decreased with increasing PC1, while the seasonality of weather increased with increasing PC1. PC2 was most related to the variability of summer temperature and winter precipitation. The increasing PC2 represented lower summer temperature and heavier winter precipitation. PC3 mainly explained the variability of summer precipitation; the value of summer precipitation was positively correlated with PC3.
Table 1.
The results of the principal component analysis of the 19 bioclimatic variables extracted from the areas of South Korea. The first three PCs with eigenvalue lager than one were represented here. The percentages in parentheses indicate the amount of variation explained by each PC, and the components that were loaded most highly for each parameter are in bold
| Variables | PC1 (56.1%) | PC2 (23.4%) | PC3 (12.1%) | |
|---|---|---|---|---|
| Bio1 | Annual mean temperature | −0.26 | −0.22 | 0.17 |
| Bio2 | Mean diurnal range | 0.26 | −0.13 | −0.14 |
| Bio3 | Isothermality | 0.14 | −0.08 | −0.26 |
| Bio4 | Temperature seasonality | 0.29 | −0.12 | 0.01 |
| Bio5 | Max temperature of warmest period | −0.10 | −0.41 | 0.20 |
| Bio6 | Min temperature of coldest period | −0.30 | −0.05 | 0.10 |
| Bio7 | Temperature annual range | 0.29 | −0.13 | −0.03 |
| Bio8 | Mean temperature of wettest quarter | −0.16 | −0.37 | 0.19 |
| Bio9 | Mean temperature of driest quarter | −0.29 | −0.05 | 0.14 |
| Bio10 | Mean temperature of warmest quarter | −0.16 | −0.36 | 0.24 |
| Bio11 | Mean temperature of coldest quarter | −0.29 | −0.10 | 0.11 |
| Bio12 | Annual precipitation | 0.01 | 0.32 | 0.45 |
| Bio13 | Precipitation of wettest period | 0.25 | 0.05 | 0.36 |
| Bio14 | Precipitation of driest period | −0.20 | 0.31 | −0.04 |
| Bio15 | Precipitation seasonality | 0.28 | −0.14 | 0.18 |
| Bio16 | Precipitation of wettest quarter | 0.20 | 0.17 | 0.43 |
| Bio17 | Precipitation of driest quarter | −0.22 | 0.30 | 0.01 |
| Bio18 | Precipitation of warmest quarter | 0.22 | 0.14 | 0.40 |
| Bio19 | Precipitation of coldest quarter | −0.22 | 0.30 | 0.02 |
| Eigenvalue | 10.7 | 4.5 | 2.3 |
Total explanation power 91.7%.
These PCs were variously associated with altitude and latitude (Fig. 3A and B). PC1 was positively correlated with both latitude (rs = 0.70) and altitude (rs = 0.51), while PC2 was strongly associated with higher altitude (rs = 0.72) but not with latitude (rs = 0.08). PC3 appeared to be negatively correlated with altitude, but the effect was weak (rs = −0.28), and no clear correlation was found with latitude (rs = −0.04). These correlations explain well the characteristics of PCs in relation to altitude and latitude, indicating that PCs fully represent proper surrogates for the environmental components considered here and the interpretation of PCs is appropriate.
Figure 3.
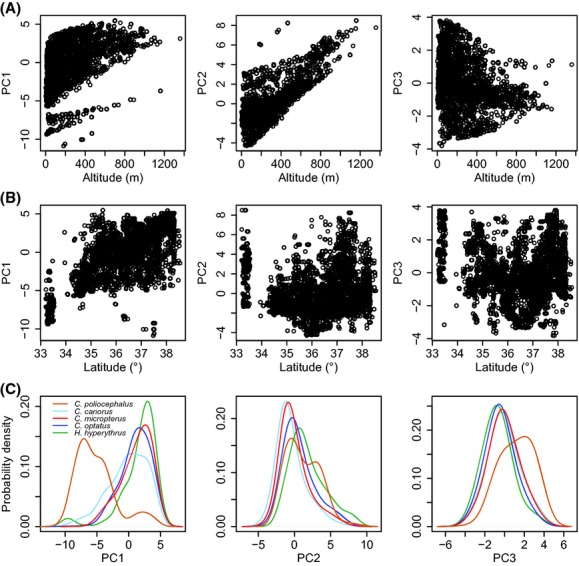
The patterns of association between the first three principal components and (A) altitude and (B) latitude. (C) The smoothed frequency distributions (kernel density plot) of the first three principal components that each species occupies.
The estimation of the probability density for PC1 showed that C. poliocephalus had a differentiated range from the other four species (Pianka index: 0.39 for C. canorus, 0.23 for C. micropterus and C. optatus, and 0.17 for H. hyperythrus; Fig. 3C). Among those four species in which the substantial range of PC1 overlapped with each other (the Pianka index ranged from 0.84 between C. canorus and H. hyperythrus to 0.98 between C. micropterus and C. optatus), C. canorus had the broadest range of PC1 (Fig. 3C). There was a tendency for sequential variation in PC2, with the broadest range for C. poliocephalus, but the overall range of PC2 also widely overlapped among the five species (the Pianka index ranged from 0.66 between C. canorus and H. hyperythrus to 0.98 between C. canorus and C. micropterus; Fig. 3C). Similar to PC1, C. poliocephalus had a differentiated range in PC3 from the other species, but the extent was smaller (the Pianka index ranged from 0.63 between C. poliocephalus and H. hyperythrus to 0.99 between C. canorus and C. micropterus). Taken together, the overall ecological niches of the five species of avian brood parasites breeding in Korea overlapped significantly more than expected by chance (Table 2).
Table 2.
The values of Pianka index (PI) showing the degree of overall niche overlap among the five species of avian brood parasites breeding in South Korea
| Mean of PI | Variance of PI | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Observed | Simulated1 | ES2 | P | Observed | Simulated | P |
| PC 1 | 0.66 | 0.50 | 3.17 | <0.01 | 0.13 | 0.03 | <0.0001 |
| PC 2 | 0.86 | 0.48 | 6.96 | <0.0001 | 0.01 | 0.03 | 0.03 |
| PC 3 | 0.86 | 0.61 | 6.12 | <0.0001 | 0.02 | 0.02 | n.s. |
| Altitude | 0.77 | 0.47 | 5.57 | <0.0001 | 0.03 | 0.03 | n.s. |
The number of iteration for simulation is 10,000.
Standardized effect size: (Observed index-Simulated index)/(Standard deviation of simulated indices).
Species distribution modeling
The average training and test AUC values for the Maxent models predicting habitat suitability for the five species of brood parasites ranged from 0.713 in C. canorus to 0.949 in H. hyperythrus and from 0.665 in C. canorus to 0.886 in H. hyperythrus, respectively (Table 3). The omission rates of test samples at 10% training sample presence also varied from 0.151 in C. canorus to 0.305 in H. hyperythrus. Overall, the model of C. canorus showed the smallest AUC value with the smallest test omission rate; in contrast, H. hyperythrus represented the largest values in AUC and test omission rate (Table 3).
Table 3.
Summary of the Maxent models for the five species of avian brood parasites breeding in South Korea. Numbers in parentheses represent the number of samples
| Species | Training AUC | Test AUC | Logistic threshold1 | Training omission1 | Test omission1 |
|---|---|---|---|---|---|
| Cuculus canorus | 0.713 (1268) | 0.665 (422) | 0.410 | 0.099 | 0.151 |
| Cuculus micropterus | 0.804 (608) | 0.751 (202) | 0.346 | 0.099 | 0.174 |
| Cuculus optatus | 0.821 (407) | 0.764 (135) | 0.356 | 0.098 | 0.182 |
| Cuculus poliocephalus | 0.925 (168) | 0.879 (55) | 0.160 | 0.095 | 0.181 |
| Hierococcyx hyperythrus | 0.949 (96) | 0.886 (31) | 0.361 | 0.094 | 0.305 |
AUC, operating characteristic curve; Number of background points are 8453.
Values at 10% training presence.
The main environmental factors that were evaluated using the jackknife test varied among species (Fig. 4). The variables related to winter precipitation (Bio14, Bio17, and Bio19) played an important role in the model prediction for C. canorus and C. micropterus. However, for C. optatus and H. hyperythrus, which were mainly observed in high mountains, altitude, and summer temperature (Alt, Bio5, Bio8, and Bio10) had large contributions to the model prediction, while the variables related to winter weather (Bio6 and Bio19) and the seasonality of temperature (Bio4 and Bio7) had large contributions to the model prediction for C. poliocephalus.
Figure 4.
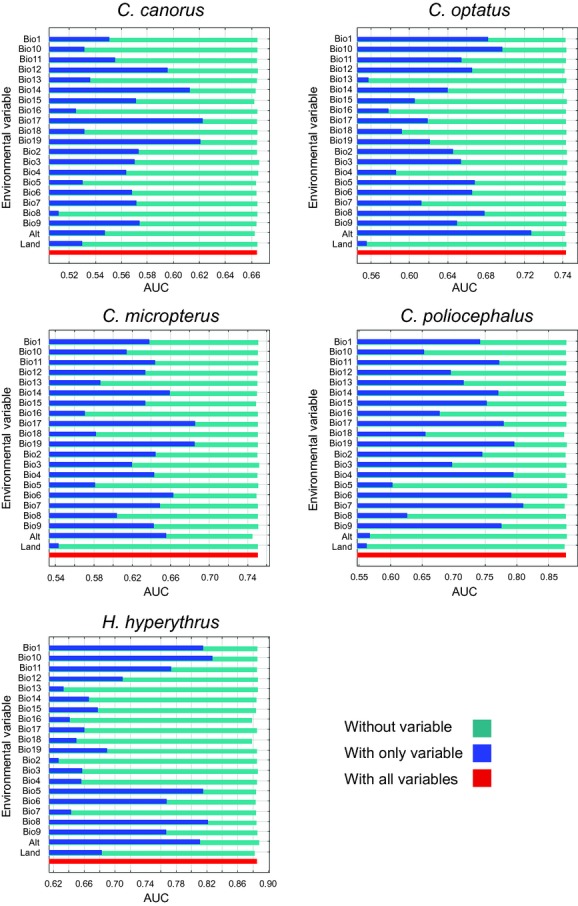
The results of the jackknife test of variable importance, using operating characteristic curve (AUC) on test data. The different colors of the bars indicate the value of AUC on test data when only a single variable is included in the Maxent model (blue) and when the variable is omitted from the model (green), respectively. The environmental variable that generates the highest AUC when used in isolation or that decreases the AUC the most when it is omitted could be considered the most useful information.
The predicted distribution areas of suitable habitats for each species largely included the actual observed distribution areas of each species (Fig. 5A). C. canorus was predicted to be able to inhabit almost all areas of mainland Korea and Jeju Island, irrespective of altitude, with higher suitability for the northern part of mainland Korea. The majority of the mountain range was predicted to be the most suitable habitat for C. optatus, C. micropterus, and H. hyperythrus, but H. hyperythrus appeared to have a much narrower range, mostly restricted to high altitude areas. Meanwhile, Jeju Island was predicted to be an inappropriate habitat for those three species, especially for C. micropterus and H. hyperythrus. In contrast, Jeju Island was expected to be the most suitable habitat for C. poliocephalus (Fig. 5A). The southern part of Korea, mainly coastal areas including many islands, also appeared to be appropriate for C. poliocephalus, while in mainland Korea, suitable habitats were patchily distributed.
Figure 5.
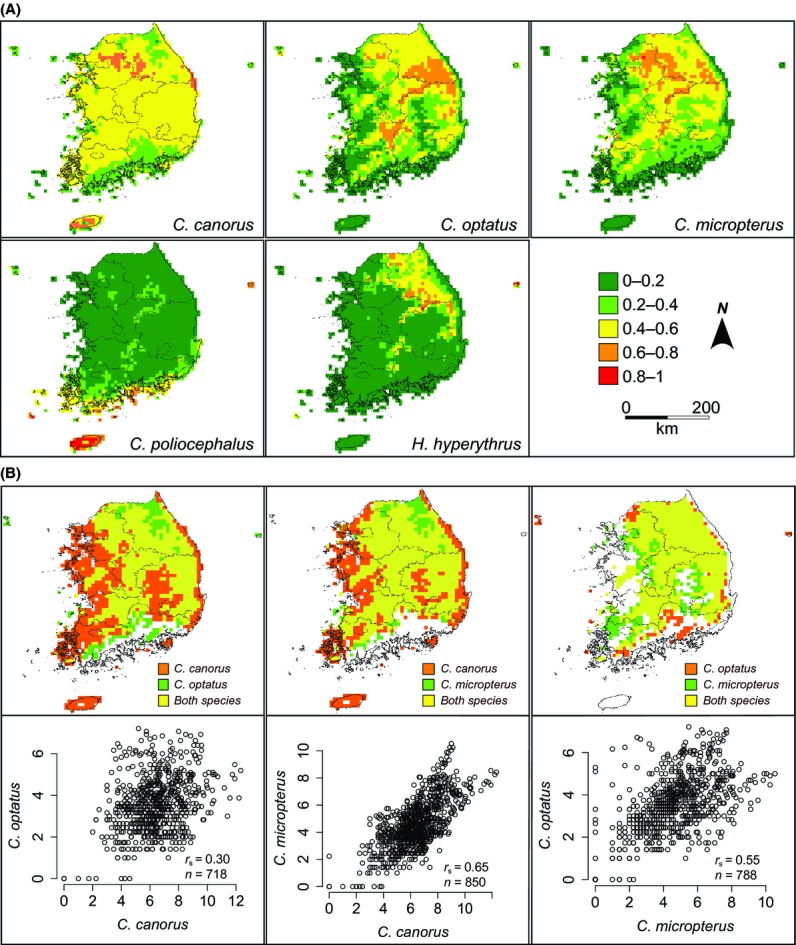
(A) Spatial prediction of the five species of avian brood parasites breeding in South Korea. Different colors represent the different degrees of occurrence probability (or habitat suitability). (B) The upper panel illustrates the overlap between predicted distributions of the three most widely distributed species in a pairwise manner. From the left panel, C. canorus versus C. optatus, C. canorus versus C. micropterus, and C. optatus versus C. micropterus, respectively. The logistic threshold of ten percentile training presence was applied to generate the presence/absence map. The orange and green colors indicate the areas where only one species was predicted to be presence while the areas with yellow color represent that both species are likely to occur together. The lower panel shows the association between the observed numbers of individuals (square-rooted) of focal species in the predicted areas (yellow) where both species occur sympatrically. The observed number of individuals was obtained from 5-min cells applying the option of circular neighborhood of 0.4 degrees.
To identify the impact of antagonistic species interactions on the distribution of species, we generated rasters of presence/absence distribution for C. canorus, C. optatus, and C. micropterus, which had similar ecological niches, by applying the threshold rule of ten percentile training presence and then those binary rasters were overlaid in pairs to locate the areas where Maxent predicted that both focal species may sympatrically occur (Fig. 5B). Then, the number of individuals observed in each cell in those areas was compared, from which we found weak to moderate positive correlations between species in their relative abundance (Fig. 5B), indicating that species interactions between brood parasites may play only a minor role in shaping the pattern of their spatial distribution.
Discussion
This study showed that the overall spatial ranges of five avian brood parasites breeding in Korea substantially overlapped with each other. Likewise, it appeared that they generally took up similar ecological niches, even though each species followed a different adaptive trajectory with respective host species. In addition, sympatric brood parasites showed positive relationships in their relative abundances, indicating that any antagonistic interaction between species may not be the first driving force shaping the pattern of the spatial distribution among avian brood parasites. Furthermore, combining these results with those of species distribution modeling confirmed again that their distribution ranges would be determined mainly by factors such as host distribution or climate conditions rather than species interactions among avian brood parasites.
Altitude is likely to be a major element characterizing the patterns of their distribution. Considering the frequency distribution of the altitude of Korea, C. canorus and C. poliocephalus were likely to be observed more frequently at lower altitudes. Alternatively, the data could also be interpreted to indicate that they tended to occur similarly across most of the range of altitudes without specific altitudinal preference, especially for C. canorus. In contrast, C. optatus, C. micropterus, and H. hyperythrus were observed more frequently at higher altitudes (>200 m) than expected by the frequency distribution of altitudes in Korea (Fig. 1C). In particular, the spatial range of H. hyperythrus was much more restricted to the higher parts of the mountain range (1st–3rd quartile: 369–602 m) than the other two species (1st–3rd quartile: C. micropterus 176–455 m; C. optatus 219–532 m). The biogeography of brood parasites should be closely coordinated with that of hosts. Thus, it is likely that these differences in altitudinal distribution may result from the altitudinal variation in the relative breeding density of their host species. For example, Paradoxornis webbianus, the primary host of C. canorus in mainland Korea, is a habitat generalist that breeds in diverse habitats at various altitudes from lowland reed beds to mountainous regions (Robson 2007). Alternatively, C. canorus may exploit different host species according to altitude because this species is known as a host generalist having many host-specific races compared to other study species. Cettia diphone, the primary host of C. poliocephalus, is also observed across various altitudinal ranges (Bairlein et al. 2006) and mainly inhabit the southern part of Korea, especially Jeju island with highest density (Lee et al. 2000), which may be one potential reason shaping the overall distribution pattern of C. poliocephalus in Korea (Fig. 1). In contrast, the potential host species of the other three species, such as Phylloscopus coronatus and Cyanoptila cyanomelana, are known to breed mainly in mountain forests (Bairlein et al. 2006; Taylor and Clement 2006). Ecological conditions such as climate and habitat types may also play a certain role in determining the altitudinal preferences of species. For example, the SDM for H. hyperythrus predicted that its range would be mainly restricted to mountain ranges with cool summer temperatures (Figs. 4, 5A), and the realized range was comparable (Fig. 1B). However, its primary host, Cyanoptila cyanomelana, may have a much broader altitudinal range than that of H. hyperythrus (Taylor and Clement 2006), indicating that host availability may not be the only factor determining the distribution of avian brood parasites. Negative species interactions between brood parasites could be an alternative factor limiting the altitudinal distribution of species, but the fact that the altitudinal ranges of all of the species substantially overlapped may diminish this probability. Anthropogenic development may be another important factor affecting not only altitudinal preference but also overall distribution pattern of species because higher altitude areas are generally less developed and thus have more natural habitats than lower areas in Korea. However, this may not be able to explain altitudinal variation of the distribution especially shown by three species (C. optatus, C. micropterus, H. hyperythrus), and we found that the effect of land cover types was small in the model (Fig. 4). Therefore, anthropogenic disturbance may affect the overall distribution pattern of species such as highest density in northeastern Korea but may not determine the altitudinal preference. Consequently, the altitudinal distribution of brood parasites seems to be closely related to altitudinal differences in ecological conditions and the distribution of their respective hosts.
In a relationship between brood parasites and hosts, niche conservatism, a tendency to retain ancestral ecological traits (Peterson et al. 1999; Wiens et al. 2010), may constrain the range of potentially available host species for brood parasites. On the other hand, antagonistic interactions between species of brood parasites may result in ecological niche differentiation between them. As with the pattern of spatial distribution, however, we found that the ecological niches of the five brood parasites were fairly similar to each other (Table 2). This implies that these closely related species tend to share ecological traits inherited from their common ancestor, even though they evolved along independent pathways of coevolution with their respective host species. In addition, contrary to expectations, our comprehensive analysis combining SDM results with observation data showed that interspecific competition between brood parasites over spatial use or host species may be trivial. However, our analysis was carried out using data extracted from a part of the entire range of the respective species. Therefore, further studies covering the additional or complete ranges of species would be worthwhile to further clarify this issue.
In SDMs, the AUC values have been widely but often uncritically used to evaluate model performance. However, it has been argued that the reliance of model evaluation on the AUC is questionable, especially when the modeling is based on presence-only data (Lobo et al. 2008; Jiménez-Valverde 2012). The AUC value is likely to be sensitive to the spatial extent of the background where pseudo-absence points are extracted; that is, increasing the area of the background may result in a larger AUC value, and vice versa. Similarly, the AUC is also influenced by the extent of distributional ranges of focal species, so the models for species with patchy distributions often generate larger AUC values than those of broadly distributed species, irrespective of sample size and actual model performance (Luoto et al. 2005; Elith et al. 2006; Hernandez et al. 2006; McPherson and Jetz 2007). In this study, the smallest training AUC value was gained from C. canorus (0.713) while the largest was from H. hyperythrus (0.949; Table 3). According to the model evaluation based solely on the AUC value, the model prediction for H. hyperythrus should be more accurate than that for C. canorus. However, the test omission rate at a 10% training presence of H. hyperythrus (0.305) was considerably larger than that of C. canorus (0.151), questioning the reliability of the AUC-based model evaluation (Table 3). Instead, we argue that the interspecific variation in the AUC values shown in this study seems to be derived from the difference in the evenness of the distribution; C. canorus was most widely distributed, while H. hyperythrus had the patchiest distribution (Fig. 1B).
In conclusion, the spatial patterns and ecological niches of avian brood parasites breeding in Korea appeared to be similar, despite potential conflicts over host availability. The results of this study imply that, contrary to expectation, interspecific competition between brood parasites may not be a primary driving force that differentiates the type of host species between brood parasites and that would ultimately lead to speciation. Alternatively, these could be achieved via somewhat indirect pathways such as host response to brood parasitism. In this study, we did not directly quantify the effect of the spatial distributions and ecological niches of host species on those of their brood parasites. Future studies including this approach will further clarify our understanding of the spatial dynamics and niche evolution in the system of avian brood parasitism. Furthermore, this aspect should be considered to be an important biotic factor in the study of future distributions according to climate change because the changing patterns of host distribution and host species composition may directly influence the spatial dynamics of brood parasites, showing the importance of biotic factors in the study of climate change, where their effects on species distribution have often been regarded as trivial.
Acknowledgments
We thank all participants who collected data on bird distribution in both surveys. We are also grateful to K.-H. Kim, W.-J. Jung and J.-Y. Lee for their help in data handling, and H.-K. Moon, K.-B. Nam and two anonymous reviewers for helpful comments. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF; www.nrf.re.kr) funded by the Ministry of Education (NRF-2012R1A6A3A04040003).
Conflict of Interest
None declared.
References
- Avilés JM, Stokke BG, Moksnes A, Røskaft E, Åsmul M. Møller AP. Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J. Evol. Biol. 2006;19:1901–1910. doi: 10.1111/j.1420-9101.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- Bairlein F, Alström P, Aymí R, Clement P, Dyrcz A, Gargallo G. Family Sylvidae (Old World Warblers) In: Del Hoyo J, Elliott A, Christie DA, editors. Handbook of the birds of the world. Volume 11. Old world flycatchers to old world warblers. Barcelona: Lynx Edicions; 2006. pp. 492–709. [Google Scholar]
- Birdlife International. 2014. IUCN Red List for birds. Available at www.birdlife.org. (accessed 15 April 2014)
- Brooke MdeL. Davies NB. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature. 1988;335:630–632. [Google Scholar]
- Davies NB. Cuckoos, cowbirds and other cheats. London: T & AD Poyster; 2000. [Google Scholar]
- Davies NB. Brooke MDL. An Experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J. Anim. Ecol. 1989;58:207–224. [Google Scholar]
- Davies NB, Madden JR, Butchart SHM. Rutila J. A host-race of the cuckoo Cuculus canorus with nestlings attuned to the parental alarm calls of the host species. Proc. Biol. Sci. 2006;273:693–699. doi: 10.1098/rspb.2005.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez S. Brood parasitism: a good strategy in our changing world? Proc. Biol. Sci. 2014;281:20132404. doi: 10.1098/rspb.2013.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunson WA. Travis J. The role of abiotic factors in community organization. Am. Nat. 1991;138:1067–1091. [Google Scholar]
- Elith J. Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009;40:677–697. [Google Scholar]
- Elith J, Graham HC, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE. Yates CJ. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011;17:43–57. [Google Scholar]
- Engler JO, Rödder D, Elle O, Hochkirch A. Secondi J. Species distribution models contribute to determine the effect of climate and interspecific interactions in moving hybrid zones. J. Evol. Biol. 2013;26:2487–2496. doi: 10.1111/jeb.12244. [DOI] [PubMed] [Google Scholar]
- Entsminger GL. EcoSim Professional: null modelling software for ecologists. Montrose, CO 81403: Acquired Intelligence Inc., Kesey-Bear, & Pinyon Publishing; 2012. http://www.garyentsminger.com/ecosim/index.htm. [Google Scholar]
- Fossøy F, Antonov A, Moksnes A, Røskaft E, Vikan JR, Møller AP, et al. Genetic differentiation among sympatric cuckoo host races: males matter. Proc. Biol. Sci. 2011;278:1639–1645. doi: 10.1098/rspb.2010.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs HL, Sorenson MD, Marchetti K, de Brooke LM, Davies NB. Nakamura H. Genetic evidence for female host-specific races of the common cuckoo. Nature. 2000;407:183–186. doi: 10.1038/35025058. [DOI] [PubMed] [Google Scholar]
- Guisan A. Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Friedman J. The elements of statistical learning-Data mining, Inference, and Prediction. New York, NY: Springer; 2009. [Google Scholar]
- Hernandez PA, Graham CH, Master LL. Albert DL. The effect of sample size and species characteristics on performance of different species distribution modelling methods. Ecography. 2006;29:773–785. [Google Scholar]
- Higuchi H. Sato S. An example of character release in host selection and egg colour of cuckoos Cuculus spp. in Japan. Ibis. 1984;126:398–404. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG. Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Hijmans RJ, Guarino L. Mathur P. 2012. DIVA-GIS Version 7.5. Manual. Available at http://www.diva-gis.org/docs/DIVA-GIS_manual_7.pdf. (accessed 14 August 2013)
- Jiménez-Valverde A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012;21:498–507. [Google Scholar]
- Jiménez-Valverde A, Lobo JM. Hortal J. Not as good as they seem: the importance of concepts in species distribution modelling. Divers. Distrib. 2008;14:885–890. [Google Scholar]
- Kang CW, Kang HM, Kim EM, Park CR. Ji NJ. A fieldguide book to the birds of Jeju. Jeju: One Tree Press; 2009. [Google Scholar]
- Kilner RM. The evolution of egg colour and patterning in birds. Biol. Rev. 2006;81:383–406. doi: 10.1017/S1464793106007044. [DOI] [PubMed] [Google Scholar]
- Kim Y. 2011. A study on the breeding ecology of Terpsiphone atrocaudata on Jeju island, Korea. [Ph.D. thesis], Jeju National University, Jeju.
- Leathwick JR. Austin MP. Competitive interactions between tree species in New Zealand’s old-growth indigenous forests. Ecology. 2001;82:2560–2573. [Google Scholar]
- Lee J-W. Yoo J-C. Effect of host egg color dimorphism on interactions between the vinous-throated parrotbill (Paradoxornis webbianus) and common cuckoo (Cuculus canorus. Korean J. Biol. Sci. 2004;8:77–80. [Google Scholar]
- Lee W-S, Koo T-H. Park J-Y. A field guide to the birds of Korea. Seoul: LG Evergreen Foundation; 2000. [Google Scholar]
- Liang W, Yang C, Stokke BG, Antonov A, FossøY F, Vikan JR, et al. Modelling the maintenance of egg polymorphism in avian brood parasites and their hosts. J. Evol. Biol. 2012;25:916–929. doi: 10.1111/j.1420-9101.2012.02484.x. [DOI] [PubMed] [Google Scholar]
- Lloyd P. Palmer AR. Abiotic factors as predictors of distribution in southern African bulbuls. Auk. 1998;115:404–411. [Google Scholar]
- Lobo JM, Jiménez-Valverde A. Real R. AUC: a misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008;17:145–151. [Google Scholar]
- Luoto M, Pöyry J, Heikkinen RK. Saarinen K. Uncertainty of bioclimate envelope models based on the geographical distribution of species. Glob. Ecol. Biogeogr. 2005;14:575–584. [Google Scholar]
- Madden JR. Davies NB. A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. Biol. Sci. 2006;273:2343–2351. doi: 10.1098/rspb.2006.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti K, Nakamura H. Gibbs HL. Host-race formation in the common cuckoo. Science. 1998;282:471–472. doi: 10.1126/science.282.5388.471. [DOI] [PubMed] [Google Scholar]
- Martin TE. Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology. 2001;82:175–188. [Google Scholar]
- McPherson MJ. Jetz W. Effects of species’ ecology on the accuracy of distribution models. Ecography. 2007;30:135–151. [Google Scholar]
- Nakamura H, Kubota S. Suzuki R. Coevolution between the common cuckoo and its major hosts in Japan: stable versus dynamic specialization on hosts. In: Rothstein SI, Robinson SK, editors; Parasitic birds and their hosts: studies in coevolution. New York, NY: Oxford Univ. Press; 1998. pp. 94–112. [Google Scholar]
- Nakamura H, Miyazawa Y. Kashiwagi K. Behavior of radio-tracked common cuckoos during the breeding season in Japan. Ornithol. Sci. 2005;4:31–41. [Google Scholar]
- Payne RB. The Cuckoos. New York, NY: Oxford Univ. Press; 2005. [Google Scholar]
- Peterson AT, Soberón J. Sánchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. [DOI] [PubMed] [Google Scholar]
- Phillips SJ. Dudík M. Modelling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175. [Google Scholar]
- Phillips SJ, Anderson RP. Schapire RE. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, Leathwick J, et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing; 2013. Available at http://www.R-project.org/. (accessed 25 September 2013) [Google Scholar]
- Ricklefs RE. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1265–1272. doi: 10.1073/pnas.0913626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson C. Family Paradoxornithidae (Parrotbills) In: Del Hoyo J, Elliott A, Christie DA, editors. Handbook of the birds of the world. Volume 12. picathartes to tits and chickadees. Barcelona: Lynx Edicions; 2007. pp. 292–320. [Google Scholar]
- Rothstein SI. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 1990;21:481–508. [Google Scholar]
- Rothstein SI. Robinson SK. Parasitic birds and their hosts: studies in coevolution. New York, NY: Oxford Univ. Press; 1998. [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL. Rice KJ. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 2009;40:415–436. [Google Scholar]
- Stokke BG, Moksnes A. Røskaft E. Obligate brood parasites as selective agents for evolution of egg appearance in passerine birds. Evolution. 2002;56:199–205. doi: 10.1111/j.0014-3820.2002.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Takasu F. A theoretical consideration on co-evolutionary interactions between avian brood parasites and their hosts. Ornithol. Sci. 2005;4:65–72. [Google Scholar]
- Taylor B. Clement P. Muscicapidae (Old World Flycatchers) In: Del Hoyo J, Elliott A, Christie DA, editors; Handbook of the birds of the world. Volume 11. Old world flycatchers to old world warblers. Barcelona: Lynx Edicions; 2006. pp. 56–163. [Google Scholar]
- Townsend Peterson A, Papeş M. Eaton M. Transferability and model evaluation in ecological niche modelling: a comparison of GARP and Maxent. Ecography. 2007;30:550–560. [Google Scholar]
- Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, et al. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 2013;88:15–30. doi: 10.1111/j.1469-185X.2012.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie I. The Cuckoo. London: Batsford; 1981. [Google Scholar]
- Yang C, Liang W, Cai Y, Shi S, Takasu F, Møller AP, et al. Coevolution in action: disruptive selection on egg colour in an avian brood parasite and its host. PLoS ONE. 2010;5:e10816. doi: 10.1371/journal.pone.0010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Liang W, Antonov A, Cai Y, Stokke BG, Fossøy F, et al. Diversity of parasitic cuckoos and their hosts in China. Chin. Birds. 2012;3:9–32. [Google Scholar]