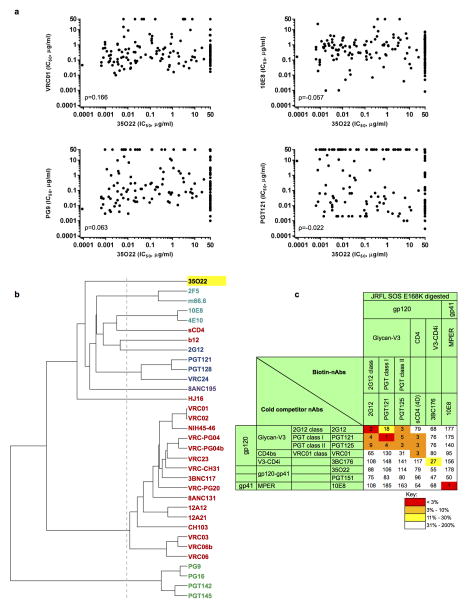
Extended Data Figure 2. Neutralization similarities between 35O22 and other HIV-1 bNAbs.
a, Correlation (Spearman) between the neutralization potencies of 35O22 and the indicated antibody against 172 pseudoviruses. Representatives from all four major sites of vulnerability are shown. Resistant strains corresponding to values of >50 μg/ml are plotted as 50. b, Neutralization-based clustering of bNabs over a set of 172 diverse HIV-1 strains. A putative epitope-specific clustering cutoff is shown as a dashed line. Antibodies are colored according to the respective target site of vulnerability: red (CD4bs), blue (glycan-V3), V1V2 (green), light blue (MPER), and other (purple). 35O22 (yellow) clusters separately from all other antibodies, indicating a novel mechanism of neutralization. c, 35O22 competition with other bNAbs on HIVJRFL VLPs with the trimer stabilizing SOS mutations in an ELISA assay. Biotin-bNAbs were titrated into the ELISA at increasing concentrations in the presence of excess (10 μg ml−1) cold competitor neutralizing antibodies. Values in the table indicates percentage binding of biotin-nAbs in the presence of cold-competitor. ND = not done