Abstract
Epitope mapping is an important tool for the development of monoclonal antibodies, mAbs, as therapeutic drugs. Recently, a class of therapeutic mAb alternatives, adnectins, has been developed as targeted biologics. They are derived from the tenth type III domain of human fibronectin (10Fn3). A common approach to map the epitope binding of these therapeutic proteins to their binding partners is X-ray crystallography. Although the crystal structure is known for Adnectin 1 binding to human EGFR, we seek to determine complementary binding in solution and to test the efficacy of footprinting for this purpose. As a relatively new tool in structural biology and complementary to X-ray crystallography, protein footprinting coupled with mass spectrometry is promising for protein-protein interaction studies. We report here the use of fast photochemical oxidation of proteins (FPOP) coupled with MS to map the epitope of EGFRAddnectin 1 at both the peptide and amino-acid residue levels. The data correlate well with the previously determined epitope from the crystal structure and are consistent with HDX MS data, which are presented in an accompanying paper. The FPOP-determined binding interface involves various amino-acid and peptide regions near the N terminus of EGFR. The outcome adds credibility to oxidative labeling by FPOP for epitope mapping and motivates more applications in the therapeutic protein area as a stand-alone method or in conjunction with X-ray crystallography, NMR, site-directed mutagenesis, and other orthogonal methods.
Introduction
Epitope mapping is an important step in the characterization of monoclonal antibodies (mAb) for their use as therapeutic drugs. Therapeutic applications of mAbs are emerging or under development for oncology, autoimmune diseases, inflammatory disorders, and organ transplantation. Recently, a new class of biologics that mimic the binding region of mAbs, Adnectins, has been developed as therapeutic mAbs analogs [1-3].
Adnectins are a class of biologics developed from the tenth human fibronectin type III domain (10Fn3), and they bind target proteins as potential therapeutic agents [1-6]. 10Fn3 is a small (10 kDa), highly stable and soluble β-sandwich protein that resembles an immunoglobulin variable domain but has no disulfide bonds [7-10]. The protein fold contains three solvent-exposed loops, termed BC, DE and FG, which are structurally analogous to antibody complementarity-determining regions (CDRs) and tolerant to extensive mutations [4]. Mutations in the loops can impart different binding capacities to the 10Fn3-based variants. Therefore, adnectins can have similar functions as therapeutic monoclonal antibodies by binding with high affinity to their targets. The first adnectin tested in preclinical and phase I studies, CT-322, targets vascular endothelial growth factor receptor-2, giving some desired pharmacological effects [11-14].
Epidermal growth factor receptor (EGFR), a member of the ErbB receptor family, mediates cell proliferation, differentiation, survival, angiogenesis, and migration [15]. EGFR has been implicated in many human cancers [16]. Its structure consists of an extracellular domain (exEGFR), a transmembrane domain, and an intracellular tyrosine-kinase domain. Cell signaling is initiated by binding of ligands, such as EGF, to exEGFR, followed by dimerization of EGFR and phosphorylation of specific EGFR tyrosine residues [15]. We recently studied the effects of dimerization and subsequent phosphorylation by mass spectrometry (MS)-based footprinting of one family member attached to a membrane [17]. Tyrosine-phosphorylated receptors serve as docking sites for intracellular signaling proteins, which initiate many signaling cascades to produce a physiological outcome [18]. This EGFR signaling network is often dys-regulated in cancer and motivates a strategy for cancer therapy with EGFR-blocking agents, including mAbs targeting exEGFR and small molecules targeting the EGFR intracellular domain [19-21].
To develop mAb alternatives for cancer therapy, a representative adnectin (Adnectin 1) that specifically binds EGFR was generated using the mRNA display technique [22, 23]. As a requirement in therapeutic drug development, the EGFR-Adnectin 1 interaction was previously characterized, in this case by X-ray crystallography, revealing the binding epitope to be distinct from those of approved mAbs [22]. Although X-ray crystallography is often regarded as the “gold standard” for structure determination, it has limitations. For example, crystallization is sometimes difficult for certain antigen-antibody complexes and requires a relatively large amount of protein. Furthermore, whether contacts from crystal structures faithfully represents biologically relevant protein-protein interfaces is questionable [24, 25]. Another approach, site-directed mutagenesis [26], is labor intensive and could cause false positives because the protein is submitted to modifications, some of which could affect structure. Binding assays [27] and PEPSCAN [28] are often restricted to linear (continuous) epitopes, and are likely to fail when facing conformational (discontinuous) epitopes, characterization of which requires maintaining the native structure of both antigen and antibody. Limited proteolysis [29] followed by MS analysis is another approach but of low resolution.
There is a growing interest in mass-spectrometry-based methods for mapping epitopes and other protein-ligand interactions. Two appropriate approaches are hydrogen-deuterium exchange (HDX) [30-36] and oxidative labeling (e.g., OH radicals, FPOP) [37-40]. These methods are sensitive, can accommodate large proteins, and can be used with the protein in native or near-native states. For example, the epitope of EGFR binding to Adnectin 1 was mapped by Iacob and Engen using HDX MS, presented in a companion paper, and the results correlate well with the structure from X-ray crystallography. Oxidative labeling has advantages because the labeling is fast, irreversible, and residue-level information can often be generated. Fast oxidative labeling (millisec time scale) was first established by Chance and coworkers [41] by radiolytic modification. Photochemical labeling initiated by laser irradiation of H2O2 was later developed by different groups [42-44]. Of those methods, photochemical oxidation of proteins (FPOP), developed by Hambly and Gross [44], uses a flow system and controls the radical life to ~ 1 μs by introducing a radical scavenger to prevent excessive labeling. Recently, Jones and Gross [40] reported the use of FPOP coupled to MS to map the conformational epitope of the serine protease thrombin and showed results that are consistent with those from HDX.
In this paper, we describe the use of FPOP to map the epitope of exEGFR-Adnectin 1 complex. To accomplish the mapping, we modified the experimental conditions from traditional FPOP settings to allow measurement in formulation-relevant conditions and to produce moderate amount of oxidation of exEGFR even in the presence of the adnectin. The FPOP data are largely consistent with the X-ray crystallography results, which show that the epitope binds at the N terminus in Domain I, as shown by decreased modifications upon Adnectin 1 binding. In addition, FPOP also revealed changes of a residue located outside the previously determined binding interface, F24, which may be due to an allosteric change in side-chain orientation that produces decreased solvent accessibility.
Experimental Section
Materials and Reagents
30% hydrogen peroxide, formic acid, trifluoroacetic acid, L-histidine, L-glutamin, L-methionine, catalase, phosphate-buffered saline, ammonium bicarbonate, dithiothreitol (DTT), iodoacetamide (IAA), and HPLC-grade solvents were purchased from Sigma Aldrich (St. Louis, MO). Trypsin/Lys-C Mix was obtained from Promega Corp. (Madison, WI). PNGase F was purchased from New England Biolabs inc (Ipswich, MA). RapiGest SF was from Waters Corp. (Milford, MA). West Nile Virus E protein Domain III (WNV E DIII) was provided by D. Fremont from Department of Pathology and Immunology at Washington University, school of medicine. Human exEGFR (residues 1-642) was expressed in Sf9 cells mammalian cells with a C-terminal His tag and purified as described previously [22]. It was supplied in phosphate buffer saline (PBS:137mM NaCl, 2.7mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4 (pH 7.2). Adnectin 1 was supplied in PBS buffer (pH 7.2). Its expression and purification were described previously [22].
FPOP labeling of Proteins
exEGFR and Adnectin 1 were mixed together at a 1:1 molar ratio at 50 μM concentration (Kd ~ 2 nM [22]) and incubated for 1 h at room temperature. FPOP labeling of proteins was performed on the same day under the same conditions. Each sample started with 5 μM protein reconstituted in 1×PBS containing 350 μM His and 20 mM H2O2. H2O2 was added just prior to infusing the solution into the tubing for FPOP. FPOP was performed as described previously [44, 45]. The KrF excimer laser power (GAM Laser Inc., Orlando, FL) was adjusted to 44 mJ/pulse, and its pulse frequency set to 6.5 Hz. The width of the laser beam at the clear sample tube window was 1.8 mm. The flow rate was adjusted to 15 μL/min to ensure a 20% exclusion volume to avoid repeated HO · exposure and reaction [43]. All collections were in vials containing 10 mM catalase and 20 mM Met to reduce left-over H2O2. For each state, exEGFR with Adnectin 1 and exEGFR alone, three separate samples were made and submitted to FPOP labeling. In addition, three control samples for exEGFR alone were handled in the same manner, but they were not laser irradiated. All samples were stored at −80 °C after solvent evaporation if they were not directly subjected to trypsin digestion.
Proteolysis
A 20 μL aliquot of each FPOP-labeled exEGFR sample was mixed with 2 μL of RapiGest SF and incubated at 80 °C for 15 min. The samples were reduced with 10 mM dithiothreitol (2 μL of 10 mM solution) at 55 °C for 30 min, alkylated with 23 mM iodoacetamide (2 μL of 300 mM solution) in the dark at RT for 30 min, and deglycosylated with 2 μL PNGsae F at 37 °C for 2 h. Solutions of 40 μL of 1×PBS and 24 μL of 0.1 M ammonium bicarbonate were then added to each aliquot to bring the pH to ~8. Samples were digested overnight with the Trypsin/Lys-C Mix at an enzyme:protein ratio of 1:25. The reaction was quenched with trifluoroacetic acid (TFA) to give a final concentration of 1%. An aliquot of 30 μL of the digested samples was cleaned up with NuTip C-18 zip tips (Glygen Corp., Columbia, MD) and eluted into 30 μL of 60% acetonitrile/40% H2O/0.1% formic acid (FA). The eluent was dried and reconstituted in 30 μL of water with 0.1% FA for autosampler loading and subsequent analysis.
Mass Spectrometry
For peptide and residue level analysis of labeled exEGFR, samples were analyzed on a LTQ Orbitrap XL (Thermo Fisher, San Jose, CA) operated in data-dependent acquisition mode. An aliquot of 5 μL of each sample was loaded by the autosampler onto and eluted from a 15 cm column with a PicoFrit tip (New Objective, Inc., Woburn, MA), custom-packed with C18 reversed phase material (Magic, 0.075 mm × 150 mm, 5 μm, 100 Å; Michrom Bioresources Inc., Auburn, CA); the chromatograph was an Ultra 1D+ UPLC (Eksigent, Dublin, CA). Peptides were eluted by a 95 min, 260 nL/min gradient coupled to the nanospray source. The gradient started with a linear increase from 2% of solvent B (CH3CN/0.1%FA) to 32% in 65 min, and then to 90% in 13 min, held at 90% B for 5 min, and re-equilibrated to solvent A (H2O/0.1%FA) for 12 min. Mass spectra were obtained at high mass resolving power (60,000, FWHH, for ions of m/z 400), and the six most abundant ions eluting per scan were subjected to CID MS2 in the ion trap, with charge-state rejection of +1 ions enabled. Precursor ions were added to a dynamic exclusion list for 12 s to ensure good sampling of each elution peak.
Adjustment of the Scavenger for FPOP
FPOP used an excimer laser to photolyze hydrogen peroxide to give two OH radicals; the H2O2 was added in low concentration to the protein solution, to form hydroxyl radicals [44, 46]. To control the radical lifetime, a pulsed laser and selected scavenger of 20 mM Gln were used for protein labeling at a microsecond time scale, faster than protein unfolding [45]. There were concerns, however, regarding the impact of high concentration of Gln on protein conformation and behavior and whether it faithfully mimicked the protein native state.
Thus, His, a common reagent in protein formulation buffers, was selected to replace Gln as a scavenger in the FPOP experiments. Because the reaction rate constant of ·OH with His is larger than that with Gln, His was expected to be a more efficient scavenger than Gln and to work at lower concentrations. To determine the concentration of His as an alternative to the previously used 20 mM Gln scavenger, each sample started with 10 μM WNV E DIII (a model protein), 20 mM H2O2 and different concentrations of His (200 μM, 350 μM, 500 μM) or 20 mM Gln in 1×PBS. The traditional FPOP condition with 20 mM Gln was included to serve as a positive control. After FPOP labeling, protein-level analysis of labeled WNV E DIII was performed on a Bruker Maxis 4G MS (Bruker Biosciences Corporation, Billerica, MA). Samples were trapped on a 2×15 mm C8 trap column (Agilent, Santa Clara, CA, USA) and desalted with a 3 min flow. A 5 min linear gradient of 4-40% CH3CN in 0.1% formic acid was used to elute the sample for protein-level analysis. The peak corresponding to +8 charge state (Figure S1) from the global mass spectrum was used for data analysis
Global analysis with the custom program afforded the fraction of unmodified protein after FPOP for all four conditions, as shown in Table 1. The table indicates that 350 μM His gave the closest scavenging ability comparing to 20 mM Gln. According to previous studies [47], one important criterion indicating the sampling of a single (native) conformation of the protein is the Poisson distribution of +0, +16, +32 species after FPOP labeling. Therefore, a more rigorous analysis was conducted, presented in the supporting information section, to model the product distribution for all four experiments. The goodness of the fit was indicated by the “Poisson distribution factor” in Table 1, for which a value closer to “1” indicated the better fit. The result also indicated the suitability of 350 μM His as a scavenger for FPOP experiments.
Table 1.
Analysis of FPOP modification on WNV E DIII at protein level with different scavengers
| Scavenger | Unmodified WNV E DIII% | Poisson distribution factor |
|---|---|---|
| 20 mM Gln | 24.9% | 0.89 |
| 500 μM His | 26.4% | 0.89 |
| 350 μM His | 26.2% | 0.91 |
| 200 μM His | 17.4% | 0.75 |
Data Analysis for FPOP labeling of exEGFR
The raw MS files were converted to .mgf files and profile mzXML files by using MassMatrix Mass Spec Data File Converter. The .mgf files of control samples were searched using MASCOT (Matrix Science, London, U.K.) against a custom-built database containing the sequence of exEGFR for sequence coverage and including deamidation at Asn or Gln, which results from N-deglycosylation in the sample handling. Oxidation at Met was also added as a variable modification. The alkylation of the samples with iodo-acetamide added a carbamidomethyl group (MW= 57.0214) to cysteines; therefore, it too was set as a fixed modification in the search. Several Asn residues were found to be entirely deamidated to form Asp or isoAsp, and they were changed in the sequence from N to D for further FPOP modification analysis. The profile mzXML files of all samples were then searched against the modified sequence for labeled and unlabeled exEGFR tryptic peptides by using ProtmapMS 2.1 (Case Western Reserve Univ., OH) with oxidative mode set to 2, meaning up to two variable modifications were allowed to occur on the same peptide. All known hydroxyl radical side-chain reaction products [48-50] were added to the modification database for searching as variable modifications. Carbamidomethyl group modification on Cys was added as a fixed modification. From the identified modifications on each tryptic peptide (Table S1, Supporting Information), the intensities of signals for the modified (Iox) and the unmodified species (I) for each peptide were then read from the raw data files. The extents of modification at peptide level were calculated by using the following equation [51]:
Quantitative analysis of oxidative labeling at residue level was also performed for regions that showed differences in extent of modification at the peptide level between exEGFR alone and exEGFR with Adnectin 1. Modification sites on the peptide were assigned with MS2 information. In a few cases, where location of a modification to a single residue wasn't possible owing to limited fragmentation information from MS2 or to the presence of interference from co-elution of peptide isomers, the modification was indicated to occur on a set of possible residues.
Results and Discussion
FPOP labeling and analysis of exEGFR
FPOP is a chemical footprinting approach that uses short-lived ·OH radicals to “snapshot” the state of a protein or a protein-complex. Hydroxyl radicals probe the solvent accessibility of the protein because its size is similar to that of water molecules, and its high reactivity allows modification of up to 14 of 20 amino acids. In the presence of a scavenger, the radical lifetime is controlled to the μs timescale, which allows fast labeling sampling presumably of a single state of the protein. In most FPOP footprinting experiments, the comparison is two-state; that is, of the same site of a protein existing in two states to reveal changes in solvent accessibility.
By introducing an irreversible modification, the FPOP-introduced label is maintained during post-labeling sample handling, making it adaptable to a typical “bottom-up” proteomics methodology of proteolysis and LC/MS/MS. This robustness is important for membrane and secreted proteins containing complex glycosylation and other modifications, which are important for the structural and functional roles of the protein [52]. The diversity of glycan modifications often adds to the difficulty of data processing in proteomics studies. In the case of exEGFR, we found that, without a deglycosylation step, trypsin digestion followed by typical database searching gave only 74% of sequence coverage. Missing were several regions between AA320 to AA550. Interestingly, these missing regions all contain a “NXT” sequence, which is the common site for N-glycosylation. Therefore, we added a deglycosylation step before trypsin digestion to bring the coverage up to 89%, yet still missing several short regions containing multiple Lys or Arg residues (Figure S2).
Epitope mapping of exEGFR-Adnectin 1
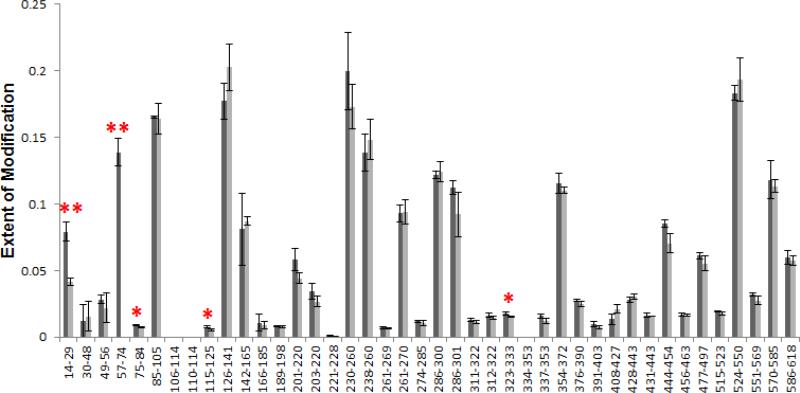
We first performed FPOP labeling of exEGFR with 350 μM His as scavenger in the absence of Adnectin 1 and analyzed the oxidation extent at the peptide level to evaluate the labeling efficiency. This is important because different proteins contain different reactive sites, and, therefore, may require different concentrations of scavengers to achieve a proper modification extent. Because exEGFR is much larger in size than WNV E DIII, EGFR is unlikely to get over-oxidized but possibly under-oxidized under the same scavenger concentration. Therefore, we only studied the oxidation extent but didn't perform distribution analysis for the peptides after FPOP labeling. The results showed that moderate oxidation, between 0% and 25%, can be achieved for all peptides (darker bars in Figure 1). This outcome is the basis for a more detailed FPOP study of the two states.
Figure 1.
Extent of modification of EGFR alone (dark bars) and Adnectin 1-bound EGFR (light bars) for tryptic peptides covering 89% of the sequence. The light bar in 57-74 is absent because the modification extent is less than 0.1%, and so are several other peptides.
When comparing the modification extent of the two states of exEGFR, we used the Student's t test on the sets of triplicates of both states for each peptide to determine if the two sets of data are significantly different from each other. The two-sample, two-tailed t test was applied assuming unequal variances, and the resulting P values for each peptide are listed in Table S2. We found a decrease in modification extent at the N-terminus for 2 of the 42 tryptic peptides of exEGFR upon Adnectin 1 binding, namely, for peptides 14-29 and 57-74 (“**” in Figure 1), of which the P values are 0.006 and 0.007, respectively. On the contrary, most of the other peptides have larger P values (Table S2), indicating no significant difference in oxidation extent between the free and bound states of exEGFR. Three other peptides, namely, 75-84, 115-125 and 323-333, also have relatively small P values (0.03 < P < 0.05) compared to most of the data (“*” in Figure 1), showing a similar trend of increased protection in the bound state. These differences are too small, however, and might be caused by structure fluctuations upon Adnectin binding rather than by interactions at the direct binding site. Noteworthy is that the modification extents for both states presented here are already adjusted by deducting the modification percentage in the control samples from them, in an effort to exclude oxidation introduced during sample handling. The reduced reactivity at those two regions indicates reduced solvent accessibility upon binding, suggesting that they are at or near the location of the binding site with Adnectin 1, assuming no allosteric effects are occurring.
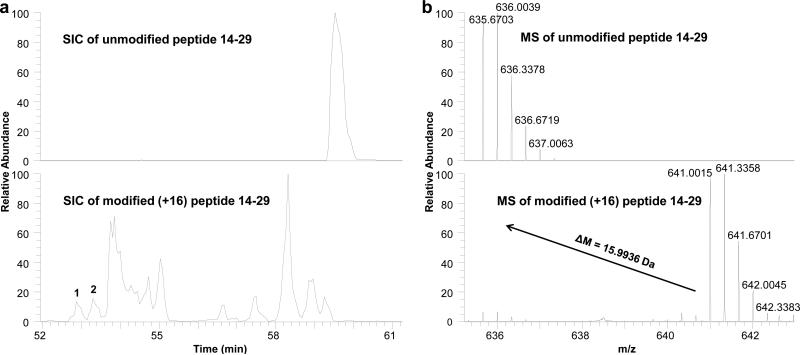
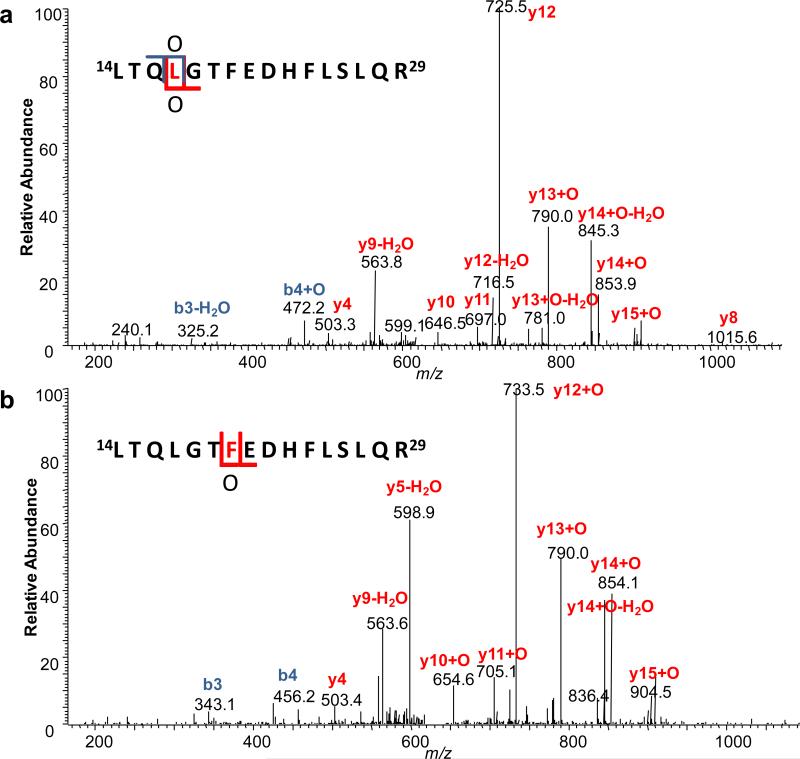
To obtain residue-specific information of the binding site, we performed residue-level analysis on the two regions that showed different modification extents for the two states. For example, the extracted ion chromatograms (EIC) and the corresponding mass spectra of peptide 14-29 (unmodified and +16 modified) are shown in Figure 2a and Figure 2b, respectively, indicating a mass shift of 15.9936 Da after modification. To assign residue-specific information to peptides eluting at each peak in the EIC, a single MS/MS scan of the +16 species at one elution time is used. An example product-ion spectrum of peptide 14-29 with a +16 modification from a single sampling (peak 1 in Figure 2a) is shown in Figure 3a. Two product ions at m/z 725.5 and 790.0, assigned to y12 and y13 +16, respectively, indicated the +16 modification is on L17. This assignment is also supported by other fragment ions, such as b3 and b4 +16. Similarly, Figure 3b shows an isomeric peptide with a +16 modification eluting at a different time (peak 2 in Figure 2a) and demonstrating that another oxidative modification occurs at a different location, here shown to be F20. In some cases, the product-ion spectrum was insufficiently informative for the assignment of the modification to a single residue. In that case, the modification was assigned to the narrowest region determined by fragment ions (e.g., region L17-H23). Worth mentioning is that positional isomers of the same residue modification are also separated in the EIC, and such peaks corresponding to the same site modification are added together for calculation.
Figure 2.
(a) Extracted ion chromatograms of peptide 14-29 unmodified (top) and +16 modified (bottom). (b) Mass spectra of peptide 14-29 normalized to 100% unmodified (top) and modified (bottom).
Figure 3.
Product-ion (MS/MS) spectrum of peptide 14-29 with +16 modification on (a) L17, colored in red and (b) F20, colored in red.
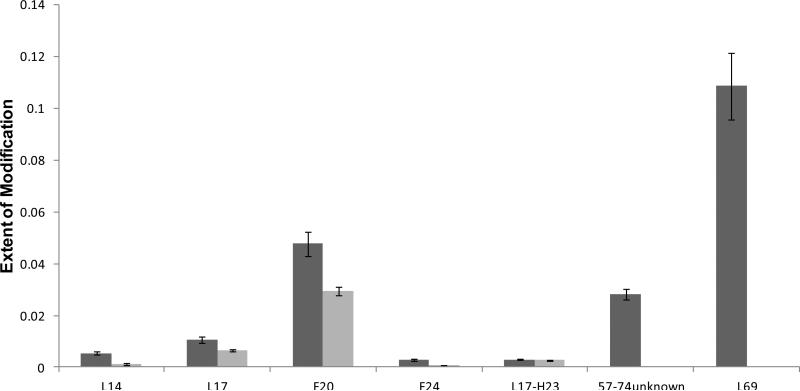
A total of seven specific residues or short regions were identified to bear the oxidations in the regions represented by peptides 14-29 and 57-74; all but one showed protection upon Adnectin 1 binding (Figure 4). A similar Student's t-test also performed on the seven residues evaluated the significance of the observed differences between the two states (Table S3). For peptide 14-29, residues L14, L17, F20, F24 were modified and showed different modification extents between the bound and unbound states (P ≤ 0.02), whereas another modification assigned to region L17-H23 showed no change between the two states (P = 0.2). In addition to the N-terminal residues, L69 from peptide 57-74, assigned with MS/MS, was also found to undergo decreased solvent accessibility and labeling extent upon Adnectin 1 binding (P = 0.03). There is another +16 modification of peptide 57-74, but we were unable to determine its location from the MS/MS information.
Figure 4.
Extent of modification of EGFR alone (dark bars) compared to Adnectin 1-bound EGFR (light bars) at the residue level for regions that showed labeling differences between the two states at peptide level.
Epitope detected by FPOP labeling vs. by other methods
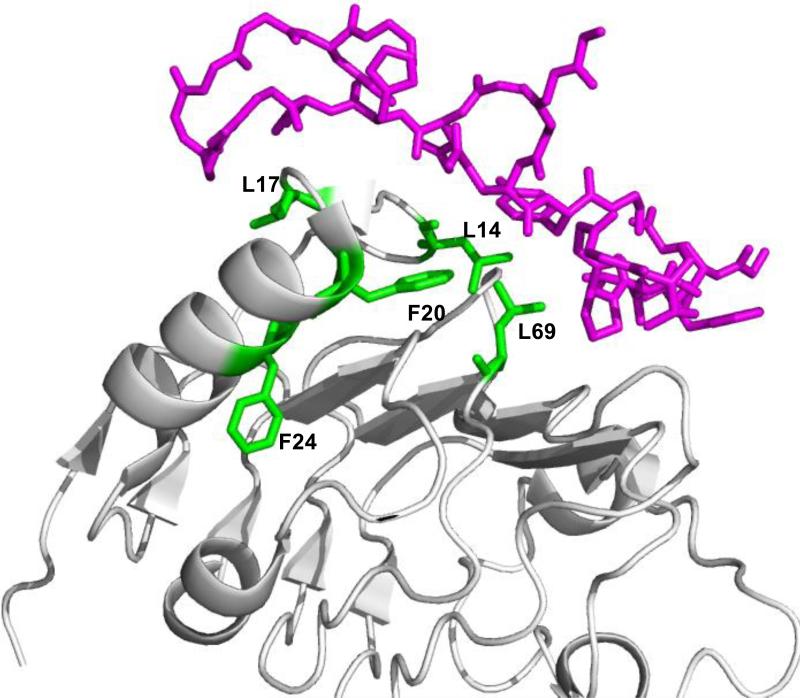
To compare the epitope of EGFR bound to Adnectin 1 as determined by FPOP labeling and crystallography, we mapped the five residues identified to undergo decreased labeling upon Adnectin 1 binding on the crystal structure of exEGFR-Adnectin 1 (PDB: 3QWQ, Figure 5). Four of the five residues, L14, L17, F20 and L69, lie at the interface of the two species when mapped onto the exEGFR-Adnectin 1 crystal structure (Figure 5). Specifically, the differential modification of L17 from FPOP labeling is consistent with the crystallography study [22], which shows that L17 interacts with K79 of Adnectin 1 via H-bonding. The crystallography also pinpoints several neighboring residues (T15, Q16 and G18) that interact with Adnectin 1 via H-bonding, and others (L14, L69, S99 and Y101) that interact via van der Waals forces. Information on T15, Q16 and G18 was not obtained, however, by FPOP labeling owing to a lack of reactivity of these residues. Although FPOP resolved interactions involving L14 and L69, it was silent concerning S99 and Y101 because these residues showed little reactivity; evidence for oxidation on those two residues was unclear because of interference from the highly oxidized M87 (located in an exposed loop) on the same peptide in the LC chromatogram. F20, although not thought to interact with ligands of EGFR, is at the buried surface of Adnectin 1 binding determined by crystallography, and, therefore, shows decreased solvent accessibility in the complex. The last residue, F24, changing its solvent accessibility as identified by FPOP, however, is distant to the putative EGFR-Adnectin 1 interface according to the crystal structure. The decrease in labeling extent on F24 may be due to a change of side-chain orientation that leads to decreased solvent accessibility or a slight change in solvent accessibly (from small to smaller) allosterically controlled by Adnectin binding.
Figure 5.
Structural model of EGFR-Adnectin 1 (PDB file 3QWQ) with the interacting residues from Adnectin 1 colored in magenta, and domain I of exEGFR, in other colors. Residues from regions 14-29 and 57-74 that showed different modification extents are in green.
When comparing the epitope mapped by HDX (in the companion paper) and by FPOP labeling, we also found good consistency. HDX MS coupled with ETD achieved epitope mapping at residue level and the most important interaction site was assigned as K13 to Q16. Noteworthy is that, unlike HDX, FPOP labeling is a measure of solvent accessibility of a side chain whereas HDX responds to changes in H-bonding of the protein backbone. Therefore, the two methods provide orthogonal information in epitope mapping, and both can complement other epitope mapping methods, such as X-ray crystallography, but reveal more relevant solution binding.
Conclusion
As a relatively new protein footprinting method, FPOP is less studied than HDX in applications involving mapping epitopes of protein antigens. In this application, we make two-state (bound and unbound) comparisons of labeling extents at identical sites. The comparisons reflect changes in solvent accessibility accompanying intermolecular interactions or allosteric changes [47]. The irreversible nature of FPOP labeling allows for complicated sample handling before MS detection, including deglycosylation, digestion, and long chromatography. This attribute is particularly important when dealing with complex samples and achieving good coverage and detailed CID analysis for residue-specific information. Here, we demonstrated the successful application of FPOP in mapping the epitope of EGFR-Adnectin 1 complex, showing largely consistent results with that from X-ray crystallography and HDX studies but revealing an additional interaction site. Five residues or short regions show decreased modification extent upon Adnectin 1 binding; of these, four lie in the binding interface determined by the crystal structure. The other (F24) is possibly caused by a change in side-chain orientation or a decrease in solvent accessibility of Adnectin 1 when bound. One advantage of MS-based protein footprinting is that it can sample physiologic mixtures. Therefore, FPOP and/or HDX can be used as stand-alone methods or in conjunction with crystallography to elucidate confidently specific binding sites of antigens in a physiological-relevant solution state.
Supplementary Material
Acknowledgements
This research was supported by the NIH, NIGMS P41 grant (P41GM103422) to MLG. The authors thank Dr. Reb Russell, Dr. Sharon Cload and Dr. Bruce Car from Bristol-Myers Squibb for their support of this project.
References
- 1.Xu L, Aha P, Gu K, Kuimelis RG, Kurz M, Lam T, Lim AC, Liu H, Lohse PA, Sun L, Weng S, Wagner RW, Lipovsek D. Directed evolution of high-affinity antibody mimics using mrna display. Chemistry & biology. 2002;9:933–942. doi: 10.1016/s1074-5521(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 2.Hackel BJ, Kapila A, Wittrup KD. Picomolar affinity fibronectin domains engineered utilizing loop length diversity, recursive mutagenesis, and loop shuffling. Journal of molecular biology. 2008;381:1238–1252. doi: 10.1016/j.jmb.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wojcik J, Hantschel O, Grebien F, Kaupe I, Bennett KL, Barkinge J, Jones RB, Koide A, Superti-Furga G, Koide S. A potent and highly specific fn3 monobody inhibitor of the abl sh2 domain. Nature structural & molecular biology. 2010;17:519–527. doi: 10.1038/nsmb.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koide A, Bailey CW, Huang X, Koide S. The fibronectin type iii domain as a scaffold for novel binding proteins. Journal of molecular biology. 1998;284:1141–1151. doi: 10.1006/jmbi.1998.2238. [DOI] [PubMed] [Google Scholar]
- 5.Koide A, Abbatiello S, Rothgery L, Koide S. Probing protein conformational changes in living cells by using designer binding proteins: Application to the estrogen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1253–1258. doi: 10.1073/pnas.032665299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karatan E, Merguerian M, Han Z, Scholle MD, Koide S, Kay BK. Molecular recognition properties of fn3 monobodies that bind the src sh3 domain. Chemistry & biology. 2004;11:835–844. doi: 10.1016/j.chembiol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type iii module of fibronectin: An insight into rgd-mediated interactions. Cell. 1992;71:671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson CD, Veerapandian B, Dai XP, Hamlin RC, Xuong NH, Ruoslahti E, Ely KR. Crystal structure of the tenth type iii cell adhesion module of human fibronectin. Journal of molecular biology. 1994;236:1079–1092. doi: 10.1016/0022-2836(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 9.Leahy DJ, Erickson HP, Aukhil I, Joshi P, Hendrickson WA. Crystallization of a fragment of human fibronectin: Introduction of methionine by site-directed mutagenesis to allow phasing via selenomethionine. Proteins. 1994;19:48–54. doi: 10.1002/prot.340190107. [DOI] [PubMed] [Google Scholar]
- 10.Leahy DJ, Aukhil I, Erickson HP. 2.0 angstrom crystal structure of a four-domain segment of human fibronectin encompassing the rgd loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 11.Mamluk R, Carvajal IM, Morse BA, Wong H, Abramowitz J, Aslanian S, Lim AC, Gokemeijer J, Storek MJ, Lee J, Gosselin M, Wright MC, Camphausen RT, Wang J, Chen Y, Miller K, Sanders K, Short S, Sperinde J, Prasad G, Williams S, Kerbel R, Ebos J, Mutsaers A, Mendlein JD, Harris AS, Furfine ES. Anti-tumor effect of ct-322 as an adnectin inhibitor of vascular endothelial growth factor receptor-2. mAbs. 2010;2:199–208. doi: 10.4161/mabs.2.2.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloom L, Calabro V. Fn3: A new protein scaffold reaches the clinic. Drug discovery today. 2009;14:949–955. doi: 10.1016/j.drudis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Molckovsky A, Siu LL. First-in-class, first-in-human phase i results of targeted agents: Highlights of the 2008 american society of clinical oncology meeting. Journal of hematology & oncology. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolcher AW, Sweeney CJ, Papadopoulos K, Patnaik A, Chiorean EG, Mita AC, Sankhala K, Furfine E, Gokemeijer J, Iacono L, Eaton C, Silver BA, Mita M. Phase i and pharmacokinetic study of ct-322 (bms-844203), a targeted adnectin inhibitor of vegfr-2 based on a domain of human fibronectin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:363–371. doi: 10.1158/1078-0432.CCR-10-1411. [DOI] [PubMed] [Google Scholar]
- 15.Janmaat ML, Giaccone G. The epidermal growth factor receptor pathway and its inhibition as anticancer therapy. Drugs of today. 2003;39(Suppl C):61–80. [PubMed] [Google Scholar]
- 16.Yarden Y, Sliwkowski MX. Untangling the erbb signalling network. Nature reviews. Molecular cell biology. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Shen W, Rempel D, Monsey J, Vidavsky I, Gross ML, Bose R. Carboxyl-group footprinting maps the dimerization interface and phosphorylation-induced conformational changes of a membrane-associated tyrosine kinase. Mol Cell Proteomics. 2011;10:M110 005678. doi: 10.1074/mcp.M110.005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 19.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer research. 1984;44:1002–1007. [PubMed] [Google Scholar]
- 20.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Molecular biology & medicine. 1983;1:511–529. [PubMed] [Google Scholar]
- 21.Flynn JF, Wong C, Wu JM. Anti-egfr therapy: Mechanism and advances in clinical efficacy in breast cancer. J. Oncol. 2009 doi: 10.1155/2009/526963. 10.1155/2009/526963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamurthy V, Krystek SR, Jr., Bush A, Wei A, Emanuel SL, Das Gupta R, Janjua A, Cheng L, Murdock M, Abramczyk B, Cohen D, Lin Z, Morin P, Davis JH, Dabritz M, McLaughlin DC, Russo KA, Chao G, Wright MC, Jenny VA, Engle LJ, Furfine E, Sheriff S. Structures of adnectin/protein complexes reveal an expanded binding footprint. Structure. 2012;20:259–269. doi: 10.1016/j.str.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Emanuel SL, Engle LJ, Chao G, Zhu RR, Cao C, Lin Z, Yamniuk AP, Hosbach J, Brown J, Fitzpatrick E, Gokemeijer J, Morin P, Morse BA, Carvajal IM, Fabrizio D, Wright MC, Das Gupta R, Gosselin M, Cataldo D, Ryseck RP, Doyle ML, Wong TW, Camphausen RT, Cload ST, Marsh HN, Gottardis MM, Furfine ES. A fibronectin scaffold approach to bispecific inhibitors of epidermal growth factor receptor and insulin-like growth factor-i receptor. mAbs. 2011;3:38–48. doi: 10.4161/mabs.3.1.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carugo O, Argos P. Protein-protein crystal-packing contacts. Protein science : a publication of the Protein Society. 1997;6:2261–2263. doi: 10.1002/pro.5560061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q, Canutescu AA, Wang G, Shapovalov M, Obradovic Z, Dunbrack RL., Jr. Statistical analysis of interface similarity in crystals of homologous proteins. Journal of molecular biology. 2008;381:487–507. doi: 10.1016/j.jmb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin DC, Berzofsky JA, East IJ, Gurd FR, Hannum C, Leach SJ, Margoliash E, Michael JG, Miller A, Prager EM, et al. The antigenic structure of proteins: A reappraisal. Annual review of immunology. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- 27.Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 28.Geysen HM, Meloen RH, Barteling SJ. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jemmerson R, Paterson Y. Mapping epitopes on a protein antigen by the proteolysis of antigen-antibody complexes. Science. 1986;232:1001–1004. doi: 10.1126/science.2422757. [DOI] [PubMed] [Google Scholar]
- 30.Yamada N, Suzuki E, Hirayama K. Identification of the interface of a large protein-protein complex using h/d exchange and fourier transform ion cyclotron resonance mass spectrometry. Rapid communications in mass spectrometry : RCM. 2002;16:293–299. doi: 10.1002/rcm.579. [DOI] [PubMed] [Google Scholar]
- 31.Baerga-Ortiz A, Hughes CA, Mandell JG, Komives EA. Epitope mapping of a monoclonal antibody against human thrombin by h/d-exchange mass spectrometry reveals selection of a diverse sequence in a highly conserved protein. Protein science : a publication of the Protein Society. 2002;11:1300–1308. doi: 10.1110/ps.4670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Witcher DR, White MA, Wang X, Huang L, Rathnachalam R, Beals JM, Kuhstoss S. Il-1beta epitope mapping using site-directed mutagenesis and hydrogen-deuterium exchange mass spectrometry analysis. Biochemistry. 2005;44:11106–11114. doi: 10.1021/bi0505464. [DOI] [PubMed] [Google Scholar]
- 33.Coales SJ, Tuske SJ, Tomasso JC, Hamuro Y. Epitope mapping by amide hydrogen/deuterium exchange coupled with immobilization of antibody, on-line proteolysis, liquid chromatography and mass spectrometry. Rapid communications in mass spectrometry : RCM. 2009;23:639–647. doi: 10.1002/rcm.3921. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Willison LN, Tripathi P, Sathe SK, Roux KH, Emmett MR, Blakney GT, Zhang HM, Marshall AG. Epitope mapping of a 95 kda antigen in complex with antibody by solution-phase amide backbone hydrogen/deuterium exchange monitored by fourier transform ion cyclotron resonance mass spectrometry. Analytical chemistry. 2011;83:7129–7136. doi: 10.1021/ac201501z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandit D, Tuske SJ, Coales SJ, E SY, Liu A, Lee JE, Morrow JA, Nemeth JF, Hamuro Y. Mapping of discontinuous conformational epitopes by amide hydrogen/deuterium exchange mass spectrometry and computational docking. Journal of molecular recognition : JMR. 2012;25:114–124. doi: 10.1002/jmr.1169. [DOI] [PubMed] [Google Scholar]
- 36.Jensen PF, Jorgensen TJ, Koefoed K, Nygaard F, Sen JW. Affinity capture of biotinylated proteins at acidic conditions to facilitate hydrogen/deuterium exchange mass spectrometry analysis of multimeric protein complexes. Analytical chemistry. 2013;85:7052–7059. doi: 10.1021/ac303442y. [DOI] [PubMed] [Google Scholar]
- 37.Takamoto K, Chance MR. Radiolytic protein footprinting with mass spectrometry to probe the structure of macromolecular complexes. Annual review of biophysics and biomolecular structure. 2006;35:251–276. doi: 10.1146/annurev.biophys.35.040405.102050. [DOI] [PubMed] [Google Scholar]
- 38.Pan Y, Brown L, Konermann L. Mapping the structure of an integral membrane protein under semi-denaturing conditions by laser-induced oxidative labeling and mass spectrometry. Journal of molecular biology. 2009;394:968–981. doi: 10.1016/j.jmb.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y, Stocks BB, Brown L, Konermann L. Structural characterization of an integral membrane protein in its natural lipid environment by oxidative methionine labeling and mass spectrometry. Analytical chemistry. 2009;81:28–35. doi: 10.1021/ac8020449. [DOI] [PubMed] [Google Scholar]
- 40.Jones LM, J BS, J AC, Gross ML. Fast photochemical oxidation of proteins for epitope mapping. Analytical chemistry. 2011;83:7657–7661. doi: 10.1021/ac2007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maleknia SD, Brenowitz M, Chance MR. Millisecond radiolytic modification of peptides by synchrotron x-rays identified by mass spectrometry. Analytical chemistry. 1999;71:3965–3973. doi: 10.1021/ac990500e. [DOI] [PubMed] [Google Scholar]
- 42.Sharp JS, Becker JM, Hettich RL. Analysis of protein solvent accessible surfaces by photochemical oxidation and mass spectrometry. Analytical chemistry. 2004;76:672–683. doi: 10.1021/ac0302004. [DOI] [PubMed] [Google Scholar]
- 43.Aye TT, Low TY, Sze SK. Nanosecond laser-induced photochemical oxidation method for protein surface mapping with mass spectrometry. Analytical chemistry. 2005;77:5814–5822. doi: 10.1021/ac050353m. [DOI] [PubMed] [Google Scholar]
- 44.Hambly DM, Gross ML. Laser flash photolysis of hydrogen peroxide to oxidize protein solvent-accessible residues on the microsecond timescale. Journal of the American Society for Mass Spectrometry. 2005;16:2057–2063. doi: 10.1016/j.jasms.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Gau BC, Sharp JS, Rempel DL, Gross ML. Fast photochemical oxidation of protein footprints faster than protein unfolding. Analytical chemistry. 2009;81:6563–6571. doi: 10.1021/ac901054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hambly D, Gross M. Laser flash photochemical oxidation to locate heme binding and conformational changes in myroglobin. Int J Mass Spectrom. 2007;259:124–129. [Google Scholar]
- 47.Gau BC, Chen H, Zhang Y, Gross ML. Sulfate radical anion as a new reagent for fast photochemical oxidation of proteins. Analytical chemistry. 2010;82:7821–7827. doi: 10.1021/ac101760y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu G, Chance MR. Radiolytic modification of acidic amino acid residues in peptides: Probes for examining protein-protein interactions. Analytical chemistry. 2004;76:1213–1221. doi: 10.1021/ac035422g. [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Chance MR. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chemical reviews. 2007;107:3514–3543. doi: 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- 50.Zhu W, Smith JW, Huang CM. Mass spectrometry-based label-free quantitative proteomics. Journal of biomedicine & biotechnology. 2010;2010:840518. doi: 10.1155/2010/840518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Gau BC, Jones LM, Vidavsky I, Gross ML. Fast photochemical oxidation of proteins for comparing structures of protein-ligand complexes: The calmodulin-peptide model system. Analytical chemistry. 2011;83:311–318. doi: 10.1021/ac102426d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of glycobiology. 2nd edition. Cold Spring Harbor Laboratories Press; NY: 2009. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.