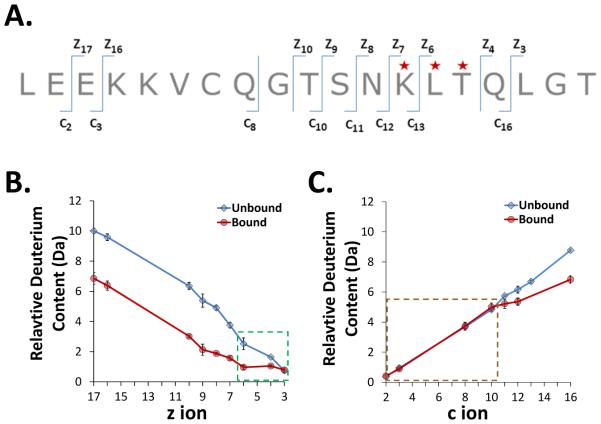
Figure 3.
Targeted ETD analysis of deuterated exEGFR bound to Adnectin 1. A. The sequence of the N-terminal exEGFR peptic peptide (residues 1-19) that was selected for ETD fragmentation, with the c and z ions that could be observed indicated. Residues highlighted with a red star showed significant differences in uptake between bound and unbound forms. Note that peptide backbone cleavage which produces c, z ions occurs on the phi bond, which is to the right (going N- to C-terminally) of the next amino acid amide hydrogen, e.g. c1 contains both the amide hydrogen for amino acid 1 and the amide hydrogen for amino acid 2 [64]. B. Deuterium content of z ions in peptide 1-19 of unbound and bound exEGFR. The green highlighted box indicates ions with differences in deuterium levels between bound and unbound forms. Notice the bound form in this region remained constant whereas uptake in the unbound form increased at higher z ion value. C. Deuterium content of c ions in peptide 1-19 of unbound and bound exEGFR. The brown highlighted box around the ions up to c10 shows the c ions which incorporated the same amount of deuterium between unbound and bound exEGFR.