Abstract
Rationale
Alcohol addiction is a chronic relapsing disorder that presents a substantial public health problem, and is frequently comorbid with posttraumatic stress disorder (PTSD). Craving for alcohol is a predictor of relapse to alcohol use, and is triggered by cues associated with alcohol and trauma. Identification of reliable and valid laboratory methods for craving induction is an important objective for alcoholism and PTSD research.
Objectives
The present study compares two methods for induction of craving via stress and alcohol cues in individuals with comorbid alcohol dependence (AD) and PTSD: the combined Trier Social Stress Test and cue reactivity paradigm (Trier/CR), and a guided imagery (Scripts) paradigm. Outcomes include self-reported measures of craving, stress, and anxiety as well as endocrine measures.
Methods
Subjects were 52 individuals diagnosed with comorbid AD and PTSD seeking treatment at the NIAAA inpatient research facility. They participated in a four week inpatient study of the efficacy of a NK1 antagonist to treat comorbid AD and PTSD, and which included the two challenge procedures.
Results
Both the Trier/CR and Scripts induced craving for alcohol, as well as elevated levels of subjective distress and anxiety. The Trier/CR yielded significant increases in ACTH and cortisol, while the Scripts did not.
Conclusions
Both paradigms are effective laboratory means of inducing craving for alcohol. Further research is warranted to better understand the mechanisms behind craving induced by stress vs. alcohol cues, as well as to understand the impact of comorbid PTSD and AD on craving.
Keywords: Alcoholism, cortisol, craving, neuroendocrine, stress, PTSD
Introduction
Alcohol is one of the leading contributors to global disease burden, and a major preventable risk factor for mortality and disability (Rehm et al. 2009). Given the relapsingremitting nature of alcohol dependence (AD), identifying relapse triggers is essential for designing effective interventions. Craving for alcohol predicts relapse (Gillespie et al. 2009; Lovallo 2006; Santa Ana et al. 2006), and both alcohol cues and stress represent potent triggers for craving and subsequent relapse in alcohol dependent individuals (Adinoff et al. 2005; Cooney et al. 1997; Higley et al. 2011; Sinha et al. 2009; Sinha et al. 2011). Thus, craving represents a useful surrogate marker for evaluating the efficacy of candidate treatments. Understanding whether different triggers for craving probe different underlying mechanisms is important for treating a disease as heterogeneous as AD. Moreover, although laboratory methodologies related to alcohol research are well-characterized (Plebani et al. 2012), there are few studies comparing these paradigms. The current study addresses this gap by comparing two methods using both stress and alcohol cues to induce craving, making an important contribution to our understanding of the mechanisms underlying craving for alcohol.
The present inquiry compares two paradigms on behavioral and physiological markers of craving, stress, and anxiety in a sample of individuals with comorbid AD and post-traumatic stress disorder (PTSD). PTSD is an anxiety disorder that is commonly comorbid with alcohol use disorders. Data from the National Comorbidity Survey indicate that among women with a lifetime history of alcohol dependence, 26% also have a lifetime history of PTSD, as do 10% of men with a lifetime history of alcohol dependence (Kessler et al. 1997). The parent study was a double-blind placebo controlled study evaluating the efficacy of an NK1 antagonist to reduce craving for alcohol (NCT00896038). All subjects were treatment-seeking alcoholics enrolled in a four-week inpatient experimental medicine study for treating comorbid AD and PTSD at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD. The results found no drug effect on craving (manuscript in preparation); thus, we collapsed the groups and included the full sample in our analyses. The parent study included neuroimaging and PTSD symptom data in addition to those presented here. The current study aims to contribute to a growing body of literature on comorbid AD and PTSD, which may result in a more severe presentation of clinical symptoms than either disorder alone, and therefore would benefit from added research. The benefits of comparing the methodologies are twofold: first, improved understanding of the physiological and behavioral correlates of craving by means of induction (stress vs. cue), and second, the ability to design interventions specific to those means of induction. As there are limited published data on either methodology in a comorbid sample, the present study draws on literature from alcohol dependent samples, and extends these to individuals with comorbid AD and PTSD.
The first method we evaluated was a combined Trier Social Stress Test (Kirschbaum et al. 1993) and cue reactivity (Monti et al. 1987) paradigm (Trier/CR). Both the Trier and the cue reactivity methods have been shown to independently affect craving. Compared with a no-stress condition, exposure to the arithmetic component of the Trier increased both placebo and alcohol consumption in social drinkers (de Wit et al. 2003), while exposure to the full Trier led to an increase in the sedative effects of alcohol in healthy volunteers (Soderpalm and de Wit 2002). The one study using the Trier in a sample with comorbid AD and PTSD found no difference in cortisol or ACTH response following the Trier between healthy controls and individuals with one or both diagnoses (McRae et al. 2006). Studies using the CR paradigm have found differences between alcoholics and non-alcoholics in the response to alcohol cues, such that alcoholics show greater physiological and subjective responses to alcohol than do non-alcoholics (Monti et al. 1987). Moreover, this increased reactivity has been associated with subsequent drinking outcomes, at least among males (Rohsenow et al. 1994). We used a combined Trier/CR, which has been shown effective in detecting increases in stress and craving (George et al. 2008) and alcohol consumed (Thomas et al. 2011a), although others have found no significant effect on craving using this methodology (Thomas et al. 2011b).
The second method in our evaluation was a guided imagery script paradigm (Scripts), which has been shown to induce both robust behavioral and physiological reactions associated with stress in alcohol dependent individuals (Sinha 2009). The paradigm includes stress, cue (associated with drug of choice), and neutral auditory scripts, to partition out contributions to craving induced by stress vs. cues. Sinha and colleagues (2009) showed that abstinent alcoholics had greater craving for alcohol, subjective distress, negative emotionality, and blood pressure compared to social drinkers in response to both stress and cue scripts, although their cortisol and heart-rate responses were relatively blunted. Additional studies have also demonstrated increases in craving and stress in response to the stress and cue scripts among treatment-seeking alcoholics (Fox et al. 2007; Sinha et al. 2011). Relatedly, Higley and colleagues (2011) demonstrated that high stress-induced craving following stress scripts was associated with shorter time to relapse, fewer percent days abstinent, higher average drinks, blunted salivary cortisol response to stress, and lower rates of abstinence in alcohol dependent outpatients.
Of further relevance to the present inquiry, Sinha and colleagues (2011) found that higher adrenal sensitivity was a predictor of shorter time to relapse in alcohol dependent subjects. These outcomes are consistent with a recent preclinical study, which showed that blockade of glucocorticoid receptors using mifepristone blocked development of compulsive alcohol self-administration in rats with a history of dependence (Vendruscolo et al. 2012), and a preliminary human laboratory study, which showed that mifepristone suppressed alcohol craving and relapse (Mason 2012). Given these findings, and those of Higley et al. (2011), and the documented links between craving and relapse, we hypothesized that ACTH and cortisol responses would negatively correlate with craving for alcohol as induced by both the Trier/CR and scripts.
Materials and Methods
Subjects
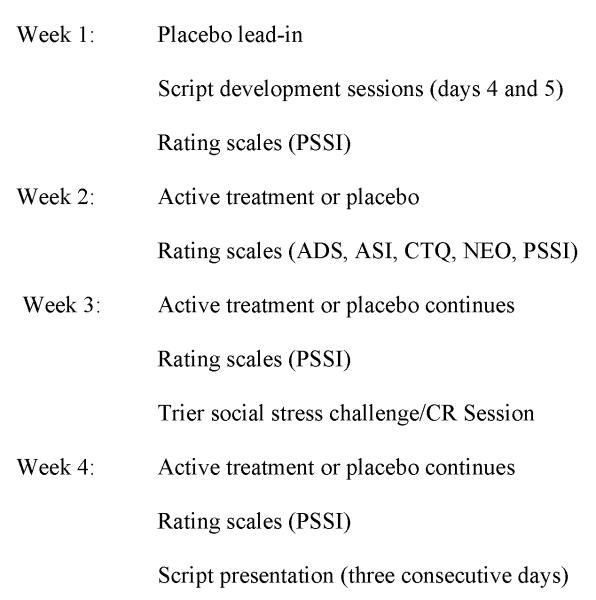
Subjects were recruited through advertisements in local media. Following a telephone screening, subjects were admitted to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) inpatient unit under an omnibus treatment and research protocol, which gathers a variety of data on subjects for screening purposes, and provides various treatment modalities. Upon admission, subjects underwent medically-managed detoxification if necessary, after which they were screened for inclusion into the current protocol. Subjects were required to be between the ages of 21 and 50, diagnosed with AD and PTSD according to the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1995) and be in good physical health. Once subjects were no longer in withdrawal, i.e., had zero breath alcohol concentration and no need for benzodiazepines to attenuate withdrawal symptoms, they underwent screening procedures to determine eligibility for participating in the protocol. These included a history and physical examination, laboratory blood tests, and the SCID. Individuals were excluded if they presented with complicated medical problems, such as advanced liver disease, were unable to participate in all study procedures, or were unable to provide informed consent. Subjects determined eligible for study underwent a capacity assessment to determine their ability to provide informed consent by independent evaluators from the NIH Department of Bioethics, following which they read and signed informed consent documents, which were approved by the NIH institutional review board. All experiments were carried out in accordance with the declaration of Helsinki. Individuals in the current study were recruited between January 2010 and January 2012. A flowchart of study procedures for Days 1 -19 of the study appears in Figure 1
Figure 1.
Timeline of study procedures.
Trier/CR Procedures
The combined Trier/CR included both a stress component and a cue presentation. The stressor comprises a social evaluative portion (public speaking to a panel of strangers) and a performance task (serial subtraction). At 3:00pm, subjects were told that they would soon be interviewing for their “dream job,” and were given 10 minutes alone with a pen and paper to prepare a five minute speech. When the time period was up, three confederates, instructed to be non-reactive, entered the room, and the participant was asked to begin the speech. After the five minute speech, the participant was instructed to being the serial subtraction task. Following that task, which also lasts five minutes, the confederates left the testing room, and blood samples and rating scales were obtained.
At 3:20pm, the cue reactivity procedure began. The participant was told that s/he would be asked to sniff two beverages, the first being water. They were instructed to hold a glass of water under their nose and to sniff it for three minutes; they were then given three minutes to relax. Following this rest period, subjects were presented with a glass and a small bottle or can containing their alcoholic beverage of choice, which they had specified at the beginning of the study. They were instructed to pour the beverage into the empty glass until two-thirds full and then hold the glass under their nose to sniff the beverage, but not drink it, for three minutes. Once this task was complete, blood samples and rating scales were obtained at pre-determined intervals over a 70 min period. The participant was observed by clinical staff via a one-way mirror while completing the CR task to ensure the participant did not drink from the glass.
Script Procedures
The script procedures comprised script construction interviews, writing, and presentation. Rajita Sinha’s group at Yale trained all script interviewers; this group also edited all scripts to ensure fidelity. During the first week of the study, subjects underwent script development interviews over two days. As subjects in the current study had PTSD, the stress scripts created referenced traumatic events; stress scripts with non-PTSD subjects are limited to stressful content occurring within a year of script construction, and are typically not traumatic (e.g., conflict in a romantic relationship, work-related disagreements, etc.). The stress script interview was conducted on the first day, and the cue and neutral scripts on the second day. No alcohol content was permitted in the stress and neutral scripts, and the cue and neutral scripts were free of stressful content. Many subjects had experienced multiple traumatic events; they were asked to describe the most traumatic event they could recall in detail. Guided imagery narratives were created from the interviews, and the final product recorded to a digital voice recorder by a male staff member with limited contact to subjects.
Scripts were presented in randomized, counterbalanced order on three consecutive days in the final week of the study. The scripts, which lasted approximately five minutes, were presented via headphones attached to digital voice recorders. The rooms in which scripts were presented were set up to afford subjects relative privacy by means of a curtain. Thus, although study personnel, including a nurse and research assistant, were present in the room during script presentation, they were not in view of the participant at that time. Study personnel participating in the script sessions did not know the order in which scripts were presented, and subjects did not know in advance prior to hearing the scripts which one they would hear on a given day. Following collection of baseline blood samples and rating scales, scripts were presented at 3pm, followed by serial blood samples and rating scales obtained over a 90-min period.
Assessments
Stress Procedures
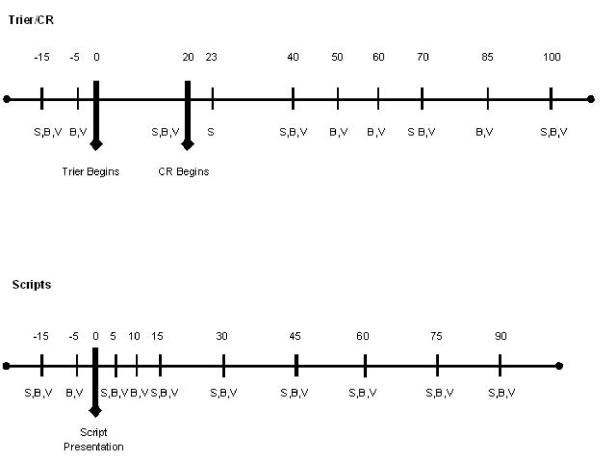
Stress response was measured using subjective and objective measures. The Subjective Units of Distress Scale (SUDS), a visual analog scale measuring from one to 100 that assesses current subjective distress, and the Spielberger State Trait Anxiety Inventory (state version, STAI-S) (Spielberger et al. 1970) were used to assess self-reported stress levels. Craving for alcohol was measured using the Alcohol Urge Questionnaire (AUQ) (Bohn et al. 1995). The AUQ is a reliable, valid instrument for assessing the subjective craving experience. Objective measures of stress response included serum levels of ACTH and cortisol. Behavioral measures, including assessments of craving, stress, and anxiety, were collected at five time points for the Trier/CR (−15, +20, +40, +70, +100 minutes) and at nine time points for the Scripts (−15, +5, +15, +30, +45, +60, +75, +90 minutes). Craving was also assessed at a +23 time point during the Trier/CR procedure, immediately following presentation of the water, and just prior to presentation of the alcohol cue, to allow for comparison of craving induced by water vs. alcohol. Endocrine measures were collected at nine time points for the Trier/CR (−15, −5, +20, +40, +50, +60, +70, +85, +100 minutes) and at 11 time points for the Scripts, (−15, −5, +5, +10, +15, +30, +45, +60, +75, +90 minutes). For both the Trier/CR and the Scripts, the 0 time point represents the initiation of the procedure, i.e., when the Trier began or when the script presentation was initiated. (See Figure 2 for a graphical representation of these time points and their relationship to study procedures.)
Figure 2.
Timeline of Trier/CR and scripts procedures.
S = scales, B = blood samples, V = vital signs.
Blood for cortisol was collected in a 3cc serum separator tube (Vacuette Z serum separator clot activator #454067) at room temperature; blood for ACTH was collected in a 3cc EDTA tube (K2 EDTA 5.4mg #367856). EDTA tubes were pre-chilled on wet ice and immediately returned to wet ice after collection. After procedures were completed, samples were sent to the NIH Department of Laboratory Medicine (CLIA ID Number 21D0665373) for immediate assay. Tests for serum cortisol were run using the IMMULITE 2000 Systems Analyzers with PIL2K/CO-20 kit, a solid-phase, competitive chemiluminescent enzyme immunoassay. The assay sensitivity was 0.20 μg/dL, with a published intraassay coefficient of variation (CV) average of 6.0% and interassay CV of 7.8%.mm. Tests for ACTH in EDTA plasma were run using the IMMULITE 2000 Systems Analyzers with PIL2KAC-15 kit, a solid-phase, two-site sequential chemiluminescent immunoassay. The assay sensitivity was 5 pg/mL, with a published intraassay CV average of 7.7% and interassay CV of 8.5%. (See figure S1 for cross-reactivity data for cortisol and ACTH).
Additional Measures
Subjects were also administered the Alcohol Dependence Scale (ADS) (Skinner and Horn 1984) to assess alcohol dependence severity and the Addiction Severity Index (ASI) (McLellan et al. 1992) to determine the overall impact of alcohol use. Additional scales administered included the PTSD Symptom Severity Index (PSSI) (Foa et al. 1993), a measure of PTSD symptom severity, the Timeline Follow-Back (TLFB) (Sobell and Sobell 1992), which assesses drinking patterns over a given time period, the NEO Personality Inventory Revised (NEO) (Costa and McRae 2002), which assesses five domains of personality (neuroticism, openness, conscientiousness, agreeableness, and extraversion), and the Childhood Trauma Questionnaire (CTQ) (Bernstein et al. 1994), which measures exposure to abusive and neglectful experiences prior to age 18.
Schedule of Procedures
The Trier/CR was carried out on approximately day 20 of the study, and the three script sessions (stress, cue, and neutral) on days 25 through 27. The Trier and CR were carried out as a combined procedure to mirror procedures from a previous study conducted by our group (George et al., 2008), and were part of the original study design. Script procedures were carried out over multiple days in accordance with the schedule established by Rajita Sinha’s group; carrying out the scripts at the same time of day over multiple days was done to minimize circadian differences in cortisol. At approximately 1:00pm on the day of challenge sessions, an in-dwelling catheter was placed, typically in the non-dominant arm, for purposes of obtaining blood samples to measure stress-related hormones. There was a 60 minute relaxation period before both challenge procedures, beginning at approximately 1:45pm. All procedures began at 2:45pm with baseline blood draws and rating scales; the challenge procedure began at 3pm (i.e., 0 time point) for both the Trier/CR and the Scripts.
Statistical Analysis
Statistical analyses addressed the following objectives: (1) to determine the effects of each procedure on craving, stress, and anxiety as a function of time, and (2) to compare the relative magnitude of response for craving, stress, and anxiety between the procedures. Data were analyzed using mixed-effect modeling and the PROC MIXED procedure in SAS version 9.3 (SAS Institute, Cary, NC). For the first set of analyses looking at response as a function of time, time point was included as the within-subjects factor, with the addition of script condition (stress, alcohol, and neutral) as a within-subjects factor for the analysis of responses to the scripting procedures. For the second set of analyses looking at the magnitude of response, peak change from baseline values were calculated for each subject under each procedure and were analyzed together in a single model with challenge procedure (Trier/CR, neutral script, alcohol script, and stress script) as the within-subjects factor. The level of statistical significance was set at p < .05 for all tests, and all post hoc comparisons were conducted using Tukey’s Honestly Significant Difference (HSD) test. Potential covariates were selected on the basis of prior literature and medical knowledge of study investigators, and were evaluated for inclusion on a model-by-model basis. Candidates included gender, treatment, race, age, ADS score, PTSD symptom severity from the PSSI, number of heavy drinking days from the TLFB, total score from the CTQ, neuroticism score from the NEO, and total score from the ASI. Further, all models included treatment status as a covariate, to control for any treatment-related effects. Model-specific covariates are noted in the relevant figure legends.
Results
Subjects
Demographics, recent drinking histories, and psychological characteristics appear in Table 1. The mean age of study subjects was 40 years (SD = 8.09, range 21 to 51), and 43% were Caucasian. Subjects drank an average of 15 drinks per day in the 90 days preceding study enrollment; alcohol dependence severity (as measured by the ADS) was in the severe range (M = 21.92, SD = 7.83). Women reported greater frequency and severity of childhood trauma than did men on the CTQ, t(50) = 2.20, p = 0.03; otherwise, there were no significant gender differences in demographic, alcohol-related, or psychosocial characteristics.
Table 1.
Participant demographics, drinking histories, and psychological characteristics.
| Female (n = 23) |
Male (n = 29) |
Total (n = 52) |
|
|---|---|---|---|
| Demographics | |||
|
| |||
| Age (M, SD) | 39.35 (8.51) | 41.90 (7.71) | 40.77 (8.09) |
| Caucasian (percent) | 43.47% | 48.15% | 43.40% |
| Years of education (M, SD) |
13.65 (2.71) | 12.86 (2.60) | 13.31 (2.62) |
| Smoker (percent) | 69.57% | 72.41% | 71.15% |
|
| |||
| Alcohol Use (past 90 days) |
|||
|
| |||
| Average Drinks/ Drinking Day |
13.22 (6.83) | 17.14 (9.4) | 15.40 (8.51) |
| Heavy Drinking Days | 62.57 (24.65) | 67.55 (23.04) | 65.35 (23.66) |
| ADS Score | 24.09 (6.55) | 20.21 (8.43) | 21.92 (7.83) |
|
| |||
| Psychological Characteristics |
|||
|
| |||
| CTQ | 59.17 (19.85) | 48.03 (16.65) | 52.96 (18.8) |
| Neuroticism | 62.10 (11.3) | 61.31 (9.9) | 61.64 (10.4) |
| PSSI | 36.35 (6.39) | 35.00 (8.7) | 35.63 (7.65) |
|
| |||
| ASI Scores | |||
|
| |||
| Employment | 0.61 (.5) | 0.66 (.48) | 0.63 (.49) |
| Family | 0.30 (.47) | 0.14 (.35) | 0.21 (.41) |
| Legal | 0.13 (.34) | 0.07 (.26) | 0.10 (.3) |
| Medical | 0.17 (.39) | 0.36 (.49) | 0.27 (.45) |
| Psych | 0.50 (.51) | 0.28 (.46) | 0.37 (.49) |
Time Course Findings
Craving
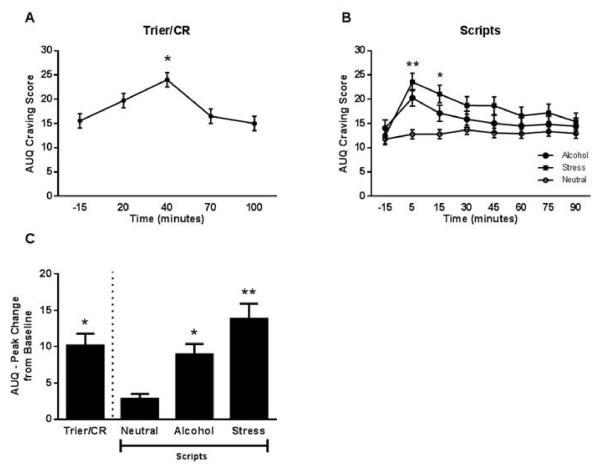
Results indicated a significant main effect of time on alcohol craving during both theTrier/CR, F (4,203) = 13.76, p < 0.0001, and Scripts, F (7, 294) = 4.73, p <0 .0001, as measured by the AUQ (Figure 3). Peak craving was observed at 40 minutes during the Trier/CR, immediately following the cue reactivity procedure, and at the 5 and 15 minute time points during the Scripts (the two time points immediately following presentation of the script). There was also a significant main effect of script type on craving, F (2,84) = 5.17, p = 0.008, with both the alcohol and stress scripts inducing higher levels of craving than the neutral script. Finally, there was a significant Script x Time interaction, F (14, 579) = 2.64, p = 0.001, such that the alcohol and stress scripts induced higher levels of craving than the neutral script at the 5 minute time point, and the stress script induced higher levels of craving than the neutral script at the 15 minute time point. There was no significant effect of treatment on craving response. Additionally, we did not find a significant correlation between craving in response to the Trier/CR or the scripts and neuroendocrine response (time-course findings for the latter appear below).
Figure 3.
Alcohol craving response to the Trier/CR and scripts.
(A) Time course of the craving response during the Trier/CR (* = different from all other time points; Tukey HSD p < 0.05). Model covariates included gender, treatment, and heavy drinking days (n = 52). (B) Time course of the craving response during the Scripts (** = alcohol and stress different from neutral, * = stress different from neutral; Tukey HSD p < 0.05). Model covariates included gender, treatment, race, PSSI severity score, and CTQ score (n = 43 due to missing data for some of the covariates). (C) Comparison of peak change from baseline craving between the Trier/CR and Scripts (* = different from neutral script, ** = different from both neutral and alcohol script; Tukey HSD p < 0.05). Model covariates included covariates included gender and treatment (n = 51). Error bars denote standard error of measurement (SEM).
Stress and Anxiety
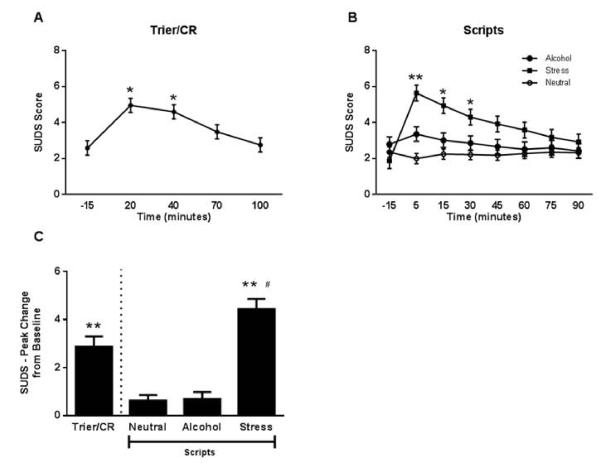
Similar patterns were found for both stress and anxiety. There was a significant main effect of time on the SUDS during the Trier/CR: F (4, 192) = 15.86., p < 0.0001 (Figure 4). During the Trier/CR, the SUDS at the 20 and 40 minute time points was significantly higher than at −15 and 100 minutes, p < 0.05, Tukey HSD post hoc. The Scripts showed significant main effects of time, F (7,343) = 3.57, p = 0.001 and script type, F (2, 98) = 6.97, p = 0.002, and a significant script by time interaction, F (14,680) = 5.37, p < 0.0001. The SUDS was higher following the stress script than the alcohol and neutrals script at the 5 and 15 minute time points, p < 0.05, Tukey HSD post hoc, and higher than the neutral script at the 30 minute time point, p < 0.05, Tukey HSD post hoc. There was no significant effect of treatment on stress response.
Figure 4.
Subjective stress response to the Trier/CR and scripts.
(A) Time course of the subjective stress response during the Trier/CR (* = different from −15, 70, and 100 minutes; Tukey HSD p < 0.05). Model covariates included gender, treatment, age, number of heavy drinking days, and neuroticism score (n = 50 due to missing data for some of the covariates). (B) Time course of the subjective stress response during the Scripts (** = different from both alcohol and neutral, * = stress different from neutral; Tukey HSD p < 0.05). Model covariates included gender, treatment, age, ADS score, and neuroticism score (n = 50 due to missing data for some of the covariates). (C) Comparison of peak change from baseline stress rating between the Trier/CR and Scripts (** = different from both alcohol and neutral script, # = different from Trier/CR; Tukey HSD p < 0.05). Model covariates included gender, treatment, and CTQ score (n = 50 due to missing data for some of the stress rating rating times, such that the peak change could not be calculated for all subjects). Error bars denote SEM.
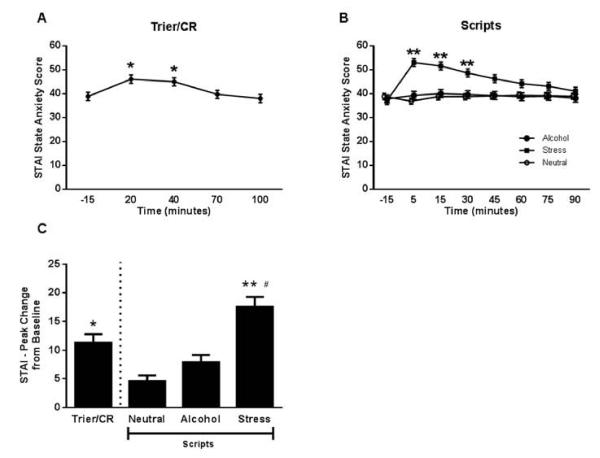
There was a significant main effect of time on the STAI-S during the Trier/CR: F (4,188) = 10.73, p < 0.0001 (Figure 5). The STAI-S at the 20 min (post-Trier) and 40 minute (post-cue reactivity) time points during the Trier/CR was significantly higher than −15, 70, and 100 minutes, p < 0.05, Tukey HSD post hoc. The Scripts showed significant main effects of time, F (7,343) = 4.8, p < 0.0001, and script type, F (2,98) = 8.80, p = 0.0003, and a significant script by time interaction, F (14,676) = 5.94, p < 0.0001. The STAI-S was higher following the stress script than the alcohol and neutral script at the 5 and 15 minute time points, p < 0.05, Tukey HSD post hoc; stress was higher following the stress script compared to the neutral script at the 30 minute time point, p < 0.05, Tukey HSD post hoc. There was no significant effect of treatment on anxiety response.
Figure 5.
Anxiety response to the Trier/CR and scripts.
(A) Time course of the anxiety response during the Trier/CR (* = different from −15, 70, and 100 minutes; Tukey HSD p < 0.05). Model covariates included gender, treatment, age, CTQ score, and neuroticism score (n = 50 due to missing data for some of the covariates). (B) Time course of the anxiety response during the Scripts (** = stress different from both alcohol and neutral; Tukey HSD p < 0.05). Model covariates included gender, treatment, age, ADS score, and neuroticism score (n = 50 due to missing data for some of the covariates). (C) Comparison of peak change from baseline anxiety between the Trier/CR and Scripts (** = different from both alcohol and neutral script, * = different from neutral script, # = different from Trier/CR; Tukey HSD p < 0.05). Model covariates included gender, treatment, and CTQ score (n = 52). Error bars denote SEM.
Neuroendocrine Function
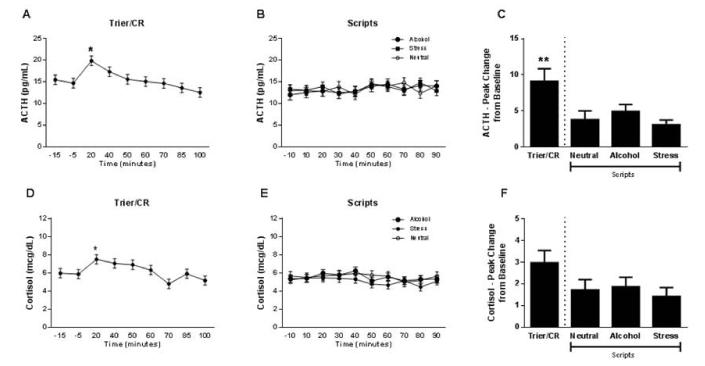
During the Trier/CR, there was a significant main effect of time on both ACTH, F (8,360) = 4.09, p = 0.0001, and cortisol, F (8,345) = 3.32, p = 0.001 (Figure 6). ACTH was significantly higher at the 20 minute time point than at all others except the 40 minute time point, p < 0.05, Tukey HSD post hoc, and cortisol was significantly higher at 20 minutes than at 70 and 100 minutes, p < 0.05, Tukey HSD post hoc. The Scripts did not elicit any significant changes in the endocrine stress response, either as a function of time or of script type. There was no significant effect of treatment on neuroendocrine response.
Figure 6.
Neuroendocrine response to the Trier/CR and scripts.
(A) Time course of the ACTH response during the Trier/CR (* = different from −15, −5, 50, 60, 70, 85 and 100 minutes; Tukey HSD p < 0.05). Model covariates included gender, treatment, and number of heavy drinking days (n = 48 due to missing data for ACTH). (B) Time course of the ACTH response during the Scripts. Model covariates included gender, treatment, age, number of heavy drinking days, and neuroticism score (n = 43 due to missing data for ACTH and for some of the covariates). (C) Comparison of peak change from baseline ACTH between the Trier/CR and Scripts (** = different from both neutral and stress script; Tukey HSD p < 0.05). Model covariates included gender, treatment and age (n = 34 due to missing data for some ACTH measurements that precluded calculation of peak change from baseline for all subjects). (D) Time course of the cortisol response during the Trier/CR (* = different from 70 and 100 minutes; Tukey HSD p < 0.05). Model covariates included gender, treatment, race ADS score, and neuroticism score (n = 46 due to missing data for some of the covariates). (E) Time course of the cortisol response during the Scripts. Model covariates included gender, treatment race, ADS score, and total score on the ASI (n = 43 due to missing data for some of the covariates). (F) Comparison of peak change from baseline cortisol between the Trier/CR and Scripts. Model covariates included gender, treatment, CTQ score, and the total score from the ASI (n = 31 due to missing data for some of the covariates and for some cortisol measurements that precluded calculation of peak change from baseline for all subjects). Error bars denote SEM.
Magnitude of Response Findings Compared Between Paradigms
Craving
Results indicated a significant effect of challenge type (Trier/CR, alcohol script, stress script, and neutral script) on peak change from baseline (PC) in craving, F(3,48) = 11.10, p < 0.0001 (Figure 3). Tukey HSD post hoc tests showed that the PC following the Trier/CR, alcohol script, and stress script were all significantly higher than the neutral script. The stress script also induced a significantly higher PC as compared to the Trier/CR.
To further compare the magnitude of response differences between paradigms, we calculated Cohen’s d, a commonly used measure of effect size, to assess two-way comparisons between the paradigms. A Cohen’s d of 0.2 is considered a small effect size; 0.5 a medium effect size; and 0.8 a large effect size. Effect sizes for all outcomes, including craving, stress, anxiety, ACTH, and cortisol appear in Table 2.
Table 2.
Effect sizes (Cohen’s d) of peak change differences between outcomes.
| Outcome | Stress Script | Cue Script | Neutral Script |
|---|---|---|---|
| AUQ | |||
|
| |||
| Stress Script | |||
| Cue Script | 0.41 | ||
| Neutral Script | 0.69 | 0.54 | |
| Trier/CR | 0.39 | 0.13 | 0.47 |
|
| |||
| SUDS | |||
|
| |||
| Stress Script | |||
| Cue Script | 0.96 | ||
| Neutral Script | 0.92 | 0.05 | |
| Trier/CR | 0.40 | 0.41 | 0.45 |
|
| |||
| STAI | |||
|
| |||
| Stress Script | |||
| Cue Script | 0.53 | ||
| Neutral Script | 0.73 | 0.25 | |
| Trier/CR | 0.40 | 0.16 | 0.42 |
|
| |||
| ACTH | |||
|
| |||
| Stress Script | |||
| Cue Script | 0.21 | ||
| Neutral Script | 0.12 | 0.06 | |
| Trier/CR | 0.43 | 0.25 | 0.29 |
|
| |||
| Cortisol | |||
|
| |||
| Stress Script | |||
| Cue Script | 0.05 | ||
| Neutral Script | 0.02 | 0.03 | |
| Trier/CR | 0.07 | 0.02 | 0.04 |
Stress and Anxiety
There was a significant effect of challenge type on PC for both stress, F(3,46) = 25.50, p < 0.0001, (Figure 4) and anxiety, F(3,48) = 13.25, p < 0.0001 (Figure 5). Tukey HSD post hoc tests showed that the PC following the Trier/CR and stress script were significantly higher than following the alcohol and neutral scripts. Further, the stress script induced significantly higher PC in stress than the Trier/CR. With respect to anxiety, the stress script induced significantly higher PC than the alcohol and neutral script, and than the Trier/CR. The Trier/CR induced significantly higher PC in anxiety as compared to the neutral script.
Neuroendocrine Function
There was a significant effect of challenge type on PC from baseline in ACTH following the Trier/CR and scripts, F(3,30) = 5.08, p = 0.006. The Trier/CR induced significantly higher PC in ACTH than the stress script. There was no significant effect of challenge type on PC in cortisol following the Trier/CR or scripts, F(3,26) = 1.64, p = 0.20.
Although no treatment effects were found in the above analyses, we conducted follow-up analyses that stratified the groups into treatment and placebo, to further probe for any effects of treatment. The majority of findings remained significant, with the exception of neuroendocrine response to the Trier/CR in the placebo group, which became non-significant when stratified. However, given that both the overall pattern is the same and that the sample size for this analysis was greatly reduced due to the stratification, these additional analyses suggest no effect of treatment on the overall findings.
Discussion
We found that both stressful and alcohol cue-related guided imagery scripts and a combined social stress – alcohol cue procedure induced alcohol craving in recently detoxified alcoholics with comorbid PTSD. Both the Trier/CR and the stressful script led to increased subjective stress and anxiety. Results of endocrine measures of stress differed between the models; the Trier/CR induced significantly elevated ACTH and cortisol in response to the challenge, while none of the scripts induced significant increases in ACTH and/or cortisol. Two important findings emerge from these data, which warrant further discussion: (1) evidence for different mechanisms underlying stress- and cue-induced craving and (2) indication that a cortisol response is not necessary for a subjective stress response. We discuss these findings and their implications below.
Our first notable finding is the evidence for dissociable mechanisms behind stress- and cue-induced craving. The observation that both the stress and cue scripts increased craving, while only the former induced anxiety, suggests the presence of distinct but convergent pathways to craving induction. Both the anxiety-related dissociation and craving-related convergence observed in our study follow closely the organization of neurocircuitry proposed to underlie stress- and drug cue-induced relapse on the basis of mapping in animal studies (Kalivas and Volkow 2005). Although functional neuroimaging studies of stress and cue-induced craving in humans have suggested overlapping neural circuits underlying both these processes, the circuitry identified in this manner, i.e. the mesolimbic and mesocortical systems (Sinha and Li 2007), have, based on preclinical literature, been postulated to map to a “final common pathway” of relapse, downstream of the dissociable stress- and cue-activated circuitry. Animal studies have been able to dissect this neurocircuitry in further detail, showing that structures of the extended amygdala are specifically involved in stress-induced reinstatement, and are upstream of this final common pathway.
Our findings were unexpected, since increased anxiety has been reported following cue scripts, and a link between alcohol cues and negative emotions has been established in alcoholics (Fox et al., 2007; Sinha et al., 2009; 2011). A major difference between these studies and the present report is the PTSD comorbidity in our sample. Because our scripts included trauma imagery, it may be useful to explore neural circuitry associated with trauma-cue exposure in individuals with PTSD. Specifically, exposure to alcohol cues leads to increased activation in the prefrontal cortex, anterior cingulate, nucleus accumbens, and anterior thalamus in alcohol dependent individuals as compared with social drinkers (Myrick et al. 2004). In contrast, a consistent pattern observed in PTSD is an exaggerated amygdala activity in response to trauma-associated stimuli, presumably due to insufficient top-down inhibition (Liberzon and Sripada 2008). Activation of the central amygdala is key for the stress-specific pathway to relapse, outlined by animal mapping studies (Kalivas and Volkow 2005). It is also critical for escalation of alcohol consumption and alcohol-seeking following a history of dependence (Heilig and Koob 2007). These observations suggest the possibility that neural substrates of PTSD and comorbid AD overlap, consistent with the extensive comorbidity between these disorders as shown by epidemiological data. An intersection between underlying neural circuitry may account for the specificity of stress-induced craving responses in this population.
A second, equally important finding of the current study is that an HPA axis activation resulting in a cortisol response is not necessary for stress-induced craving, as evidenced by the significant increase in craving following the stress script, absent a significant increase in cortisol. This finding suggests that while glucocorticoids over time may drive plasticity that promotes compulsive drinking (Vendruscolo et al. 2012), behavioral stress responses that acutely trigger craving and relapse, such as craving or negative emotionality, do not require glucocorticoid activity. This is in agreement with animal studies, which have demonstrated that stress-induced reinstatement of alcohol seeking is not influenced by adrenalectomy followed by replacement treatment with constant levels of exogenously administered glucocorticoid (Le et al. 2000).
In contrast, a prior study in abstinent alcoholics found a significant increase in salivary cortisol in response to the cue script as compared to the neutral and stress scripts; ACTH was not measured in this study (Fox et al. 2007). This study also found significantly higher anxiety in the cue script than in the neutral; thus, the endocrine response was explained as a possible combination of appetitive desire for alcohol, anxiety, and negative affect (sadness and fear). This rationale may also explain the time point associated with peak endocrine response during the Trier/CR. This time point (20 minutes) comes just after the stressor, but just prior to the CR. Given that subjects knew they would be presented with their preferred alcoholic beverage, this expectancy may have contributed to the increased endocrine response during this procedure. However, we did not find support for our hypothesis predicting correlations between craving and neuroendocrine response. Given prior findings that blunted neuroendocrine response predicted alcohol consumption (Sinha et al., 2011) it is also possible that this response may be specifically related to relapse, or at least suggests a complex relationship between HPA axis response and craving, as discussed above.
The Trier/CR is a social stressor that contains an element of novelty; both are known to activate the HPA axis. In contrast, the Scripts procedure involved guided imagery of a familiar event. A recent study of healthy volunteers comparing responses to Trier procedures conducted with an unsupportive audience, a supportive audience, or no audience at all found that the presence of others, even those rated as supportive, increased cortisol, heart rate, and blood pressure responses more than the no audience condition (Taylor et al. 2010). These findings suggest that the social component of a stressor may be important for inducing a neuroendocrine stress response. In this context, a recent neuroimaging study has shown increased responses to a social stressor in alcoholics (Maurage et al. 2012). Lastly, subjects are asked to remain standing for the 10 minute duration of the Trier/CR, while they remain seated during the scripts. Although studies of the effects of postural changes on cortisol are equivocal (Hennig et al. 2000; Hucklebridge et al. 2002), it is possible that this difference contributed to our results.
The fact that our sample included individuals with comorbid AD and PTSD may be relevant for the differential neuroendocrine results obtained in the two craving induction models. Both disorders are robustly associated with HPA axis dysfunction. Alcohol dependence is primarily associated with lower levels of basal cortisol and low responsiveness to a stressor (Adinoff et al. 2005; Lovallo et al. 2000; Sinha et al. 2009). PTSD has been similarly associated with low basal cortisol and blunted neuroendocrine response to challenge (Santa Ana et al. 2006), although variable results have also been reported (Bremner et al. 2003; Elzinga et al. 2003; Liberzon et al. 1999). The limited research done on HPA axis function in individuals with comorbid PTSD and AD has yielded mixed results. Several studies comparing individuals diagnosed with AD, PTSD, or both conditions to healthy individuals found attenuated ACTH responses in all three patient groups compared to the control group in response to the cold pressor task (CPT) (Brady et al. 2006), but not the Trier (McRae et al. 2006). Because our study only evaluated subjects with comorbid AD and PTSD, we cannot directly speak to whether the HPA axis responses we observed differ from healthy controls, or patients with one but not both of the disorders. However, serum cortisol measures are tightly calibrated, allowing a comparison across studies. This calibration allows a comparison of responses in our study compared to those obtained by Brady and colleagues. Relatedly, it is possible that our inability to detect a relationship between neuroendocrine and craving responses may have been due to the overall low neuroendocrine response to the stressors, i.e., there was too little variation to detect a relationship.
Limitations
Our sample represents a specific subset of alcohol dependent individuals: treatment-seeking subjects with comorbid PTSD. Thus, generalizability is somewhat limited. Additionally, this comorbidity may have had an impact on their reaction to both study procedures, rendering them potentially more reactive to stressors than alcoholics with low levels of anxiety. Given that the order in which the Trier and CR were administered during the combined procedure was the same for all subjects, we have no way to test order effects that may have occurred. The same is true for any order effects that may have existed between procedures; the Trier/CR always occurred prior to the Scripts, and it is possible that this order may have had an impact on the lack of neuroendocrine response during the Scripts. Further, the combination of the Trier and CR makes it difficult to assess the significance of the time point occurring in between the two, as it could be related to either effects of the stressor or anticipation of the cue. The lack of a neutral cue during the Trier/CR limits its direct comparability with the Scripts. The Trier/CR procedure had been used successfully by our group (e.g., George et al., 2008) in the past, so was not altered for the current study, the original purpose of which was to test a novel therapeutic for treatment of comorbid PTSD and AD. Finally, although we did not see a treatment effect, it is possible that administration of the NK1 antagonist may have impacted our findings, an outcome for which we attempted to control by including treatment as a covariate in all analyses, with no impact on results.
Future Directions
The findings of the current study suggest several points for future research. First, the dissociation between HPA axis activity and craving for alcohol must be explored further. Increased knowledge of this dissociation will allow us to better understand the neurobiology of craving, in ways potentially helpful to medications development. Second, the differences in craving mechanisms demonstrated by the results, i.e., script-specific cravings related to both cues and stress, and craving immediately following cue presentation but not following the social stressor in the Trier/CR, suggest a dissociation between these mechanisms of craving induction. Further research must also consider that stress and alcohol cues are often correlated in real-world contexts, rendering important studies that permit assessment of their potential interactions. Understanding these will permit targeted pharmacological and behavioral interventions aimed at reducing craving and preventing relapse.
Supplementary Material
Acknowledgements
Funded by the Division of Intramural Clinical and Biological Research, NIAAA. Thanks to Lauren Adams, Jessica Berman, Eric Markey, Victoria Brown, Keva Garg, and Byung Joon Park; Kristie Diamond, Debra Hill, Cheryl Jones, Monte Philips, and Erick Singley; and James Paterson, Judie Jones, Mary Ley, Jacqueline Goodson, and the 1SE Nursing Staff of the NIH Clinical Center.
This research was supported by the Division of Intramural Clinical and Biological Research, NIAAA, NIH.
Footnotes
The authors declare no conflicts of interest.
Authors Contribution MH, DG, DH, VR, and RS were responsible for the study concept and design. LK and JS contributed to data collection. MS analyzed the data, and LK assisted. LK drafted the manuscript. MH, DG, VR, RS, and MS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall PK. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67:700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Costa PT, McRae AL. NEO Personality Inventory-Revised (NEO PI-R) APA; Washington, DC: 2002. [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition. Biometric Research; New York: 1995. [Google Scholar]
- Foa E, Riggs D, Dancu C, Rothbaum B. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–474. [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Friebe J, Ryl I, Kramer B, Bottcher J, Netter P. Upright posture influences salivary cortisol. Psychoneuroendocrinology. 2000;25:69–83. doi: 10.1016/s0306-4530(99)00037-2. [DOI] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 2011;218:121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklebridge F, Mellins J, Evans P, Clow A. The awakening cortisol response: no evidence for an influence of body posture. Life Sci. 2002;71:639–646. doi: 10.1016/s0024-3205(02)01726-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Amer J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Mason BJ. A human laboratory study of mifepristone treatment for alcohol dependence. Annual Meeting of the American College of Neuropsychopharmacology; Hollywood, FL. 2012. [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P. Disrupted regulation of social exclusion in alcohol-dependence: an FMRI study. Neuropsychopharmacology. 2012;37:2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res. 2012;36:455–466. doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Wunschel SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, LaRowe SD, Timmerman MA, Upadhyaya H, Brady KT. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31:501–509. doi: 10.1016/j.psyneuen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Skinner H, Horn J. Alcohol Dependence Scale: User’s Guide. Addiction Research Foundation; Toronto: 1984. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self reported ethanol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa: 1992. pp. 41–72. [Google Scholar]
- Soderpalm AH, de Wit H. Effects of stress and alcohol on subjective state in humans. Alcohol Clin Exp Res. 2002;26:818–826. doi: 10.1097/00000374-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1970. [Google Scholar]
- Taylor SE, Seeman TE, Eisenberger NI, Kozanian TA, Moore AN, Moons WG. Effects of a supportive or an unsupportive audience on biological and psychological responses to stress. J Pers Soc Psychol. 2010;98:47–56. doi: 10.1037/a0016563. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 2011a;218:19–28. doi: 10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall PK, Brady K, See RE, Drobes DJ. An acute psychosocial stressor does not potentiate alcohol cue reactivity in non-treatment-seeking alcoholics. Alcohol Clin Exp Res. 2011b;35:464–473. doi: 10.1111/j.1530-0277.2010.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.