Abstract
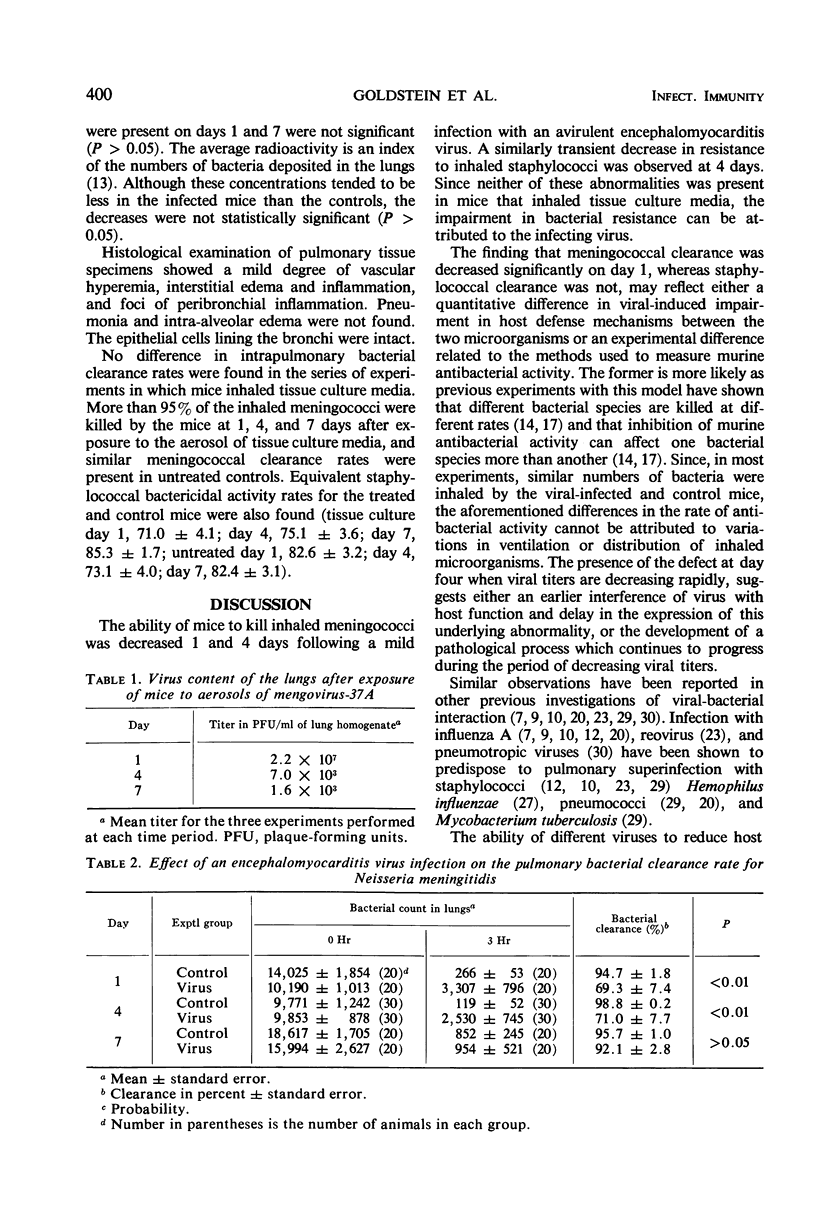
A reduction in pulmonary anti-bacterial activity due to a preceding viral illness has been suggested as the mechanism responsible for some meningococcal infections of the lung. We investigated this proposed pathogenesis by infecting mice with airborne encephalomyocarditis virus (EMC) and then challenging them 1, 4, and 7 days later with aerosols of Neisseria meningitidis. Meningococcal clearance was assessed by comparing the numbers of bacteria present immediately after inhaling the aerosols with the numbers present 3 hr later. To insure that EMC virus adequately depressed murine defense mechanisms, we also determined staphylococcal killing rates at 4 hr by using radiophosphorus-labeled staphylococcal aerosols. Viral infection depressed murine pulmonary antimeningococcal activity at 1 and 4 days (P < 0.01) but not at 7 days. Intrapulmonary staphylococcal killing was impaired on day 4 (P < 0.01) but not on days 1 or 7. Pulmonary viral titers decreased rapidly from 107 to 103 plaque-forming units/ml of lung during the experimental period. According to these data viral disease transiently depresses resistance to meningococcal infection. This impairment in host resistance is present while the viral titer is decreasing and follows a relatively similar pattern to the transient decrease noted for staphylococci.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers T. G., Bond S. B., Papke C., Leif W. R. Virulence and immunogenicity in mice of airborne encephalomyocarditis viruses and their infectious nucleic acids. J Immunol. 1966 Sep;97(3):379–385. [PubMed] [Google Scholar]
- Akers T. G., Bond S., Goldberg L. J. Effect of temperature and relative humidity on survival of airborne Columbia SK group viruses. Appl Microbiol. 1966 May;14(3):361–364. doi: 10.1128/am.14.3.361-364.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers T. G., Madin S. H., Schaffer F. L. The pathogenicity in mice of aerosols of encephalomyocarditis group viruses or their infectious nucleic acids. J Immunol. 1968 Jan;100(1):120–127. [PubMed] [Google Scholar]
- Artenstein M. S., Rust J. H., Jr, Hunter D. H., Lamson T. H., Buescher E. L. Acute respiratory disease and meningococcal infection in army recruits. JAMA. 1967 Sep 25;201(13):1004–1007. [PubMed] [Google Scholar]
- Eickhoff T. C. Sero-epidemiologic studies of meningococcal infection with the direct hemagglutination test. J Infect Dis. 1971 May;123(5):519–526. doi: 10.1093/infdis/123.5.519. [DOI] [PubMed] [Google Scholar]
- GARBER E. D., HAUTH F. C. A new mutation with asymmetrical expression in the mouse. J Hered. 1950 May;41(5):122–124. doi: 10.1093/oxfordjournals.jhered.a106105. [DOI] [PubMed] [Google Scholar]
- GERONE P. J., WARD T. G., CHAPPELL W. A. Combined infections in mice with influenza virus and Diplococcus pneumoniae. Am J Hyg. 1957 Nov;66(3):331–341. doi: 10.1093/oxfordjournals.aje.a119906. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Green G. M. Alteration of the pathogenicity of Pasteurella pneumotropica for the murine lung caused by changes in pulmonary antibacterial activity. J Bacteriol. 1967 May;93(5):1651–1656. doi: 10.1128/jb.93.5.1651-1656.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- Green G. M., Kass E. H. The influence of bacterial species on pulmonary resistance to infection in mice subjected to hypoxia, cold stress, and ethanolic intoxication. Br J Exp Pathol. 1965 Jun;46(3):360–366. [PMC free article] [PubMed] [Google Scholar]
- Green G. M. Patterns of bacterial clearance in murine influenza. Antimicrob Agents Chemother (Bethesda) 1965;5:26–29. [PubMed] [Google Scholar]
- Green L. H., Green G. M. Differential suppression of pulmonary antibacterial activity as the mechanism of selection of a pathogen in mixed bacterial infection of the lung. Am Rev Respir Dis. 1968 Nov;98(5):819–824. doi: 10.1164/arrd.1968.98.5.819. [DOI] [PubMed] [Google Scholar]
- Hahn F. F., Goldstein E., Dungworth D. L. Effect of whole-body x-irradiation on pulmonary bactericidal function. Radiat Res. 1971 Aug;47(2):461–471. [PubMed] [Google Scholar]
- Kilburn K. H. Cilia and mucus transport as determinants of the response of lung to air pollutants. Arch Environ Health. 1967 Jan;14(1):77–91. doi: 10.1080/00039896.1967.10664699. [DOI] [PubMed] [Google Scholar]
- Klein J. O., Green G. M., Tilles J. G., Kass E. H., Finland M. Effect of intranasal reovirus infection on antibacterial activity of mouse lung. J Infect Dis. 1969 Jan;119(1):43–50. doi: 10.1093/infdis/119.1.43. [DOI] [PubMed] [Google Scholar]
- LAURENZI G. A., BERMAN L., FIRST M., KASS E. H. A QUANTITATIVE STUDY OF THE DEPOSITION AND CLEARANCE OF BACTERIA IN THE MURINE LUNG. J Clin Invest. 1964 Apr;43:759–768. doi: 10.1172/JCI104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOURIA D. B., BLUMENFELD H. L., ELLIS J. T., KILBOURNE E. D., ROGERS D. E. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J Clin Invest. 1959 Jan;38(1 Pt 2):213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putsch R. W., Hamilton J. D., Wolinsky E. Neisseria meningitidis, a respiratory pathogen? J Infect Dis. 1970 Jan;121(1):48–54. doi: 10.1093/infdis/121.1.48. [DOI] [PubMed] [Google Scholar]
- SELLERS T. F., Jr, SCHULMAN J., BOUVIER C., McCUNE R., KILBOURNE E. D. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961 Aug 1;114:237–256. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S. W., Adler J. L., Sullivan R. J., Jr, Marine W. M. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969. Arch Intern Med. 1971 Jun;127(6):1037–1041. [PubMed] [Google Scholar]
- WALSH J. J., DIETLEIN L. F., LOW F. N., BURCH G. E., MOGABGAB W. J. Bronchotracheal response in human influenza. Type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Arch Intern Med. 1961 Sep;108:376–388. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]


