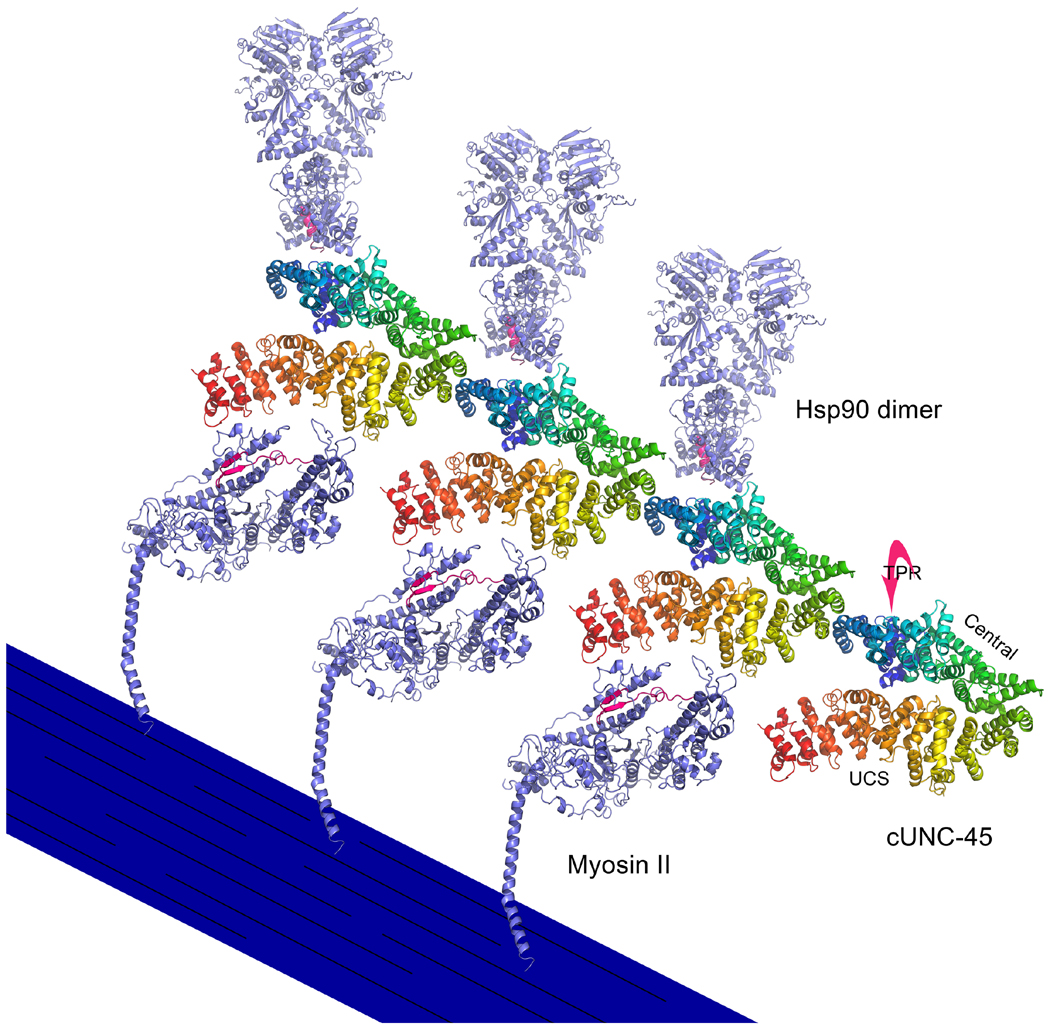
Figure 8. UNC-45 filament structure and its putative interactions with myosin S1 and Hsp90.
Based upon its crystal structure configuration, C. elegans UNC-45 (cUNC-45) forms filaments as a result of interactions between TPR domains and central/UCS neck regions of adjacent molecules (Gazda et al., 2013). The UCS domains of these filaments are proposed to interact with the periodically-spaced S1 heads of myosin thick filaments to facilitate their folding (Gazda et al., 2013). The region of muscle myosin II that may interact with UNC-45 is colored in red, based upon the ability of the homologous region of myosin V to interact directly with the She4p UCS protein in vitro (Shi and Blobel, 2010). The C-terminal peptide (MEEVD) of Hsp90 interacts with the TPR domain of cUNC-45, which lies behind the central domain as depicted (Gazda et al., 2013). The C-terminus of one Hsp90 subunit of the homodimer is colored red to show the area of possible interaction with cUNC-45. It is hypothesized that the UNC-45 filament serves as a scaffold to present Hsp90 chaperone activity to the UCS-bound unfolded myosin S1 domains (Gazda et al., 2013). In this diagram, a stylized thick filament displays folded myosin heads that have detached from cUNC-45. The Hsp90 molecules have detached from cUNC-45 as well. cUNC-45 (PDBID 4I2Z), myosin II (PDBID 2MYS), hsp90 (PDBID 2CG9) are modeled in PyMOL. Protein structures are modeled in PyMOL (PyMOL Molecular Graphics System, Version 1.6.0.0 Schrödinger LLC).