Abstract
Unrelated cord blood transplantation (CBT) is an alternative treatment option for patients who lack a matched donor. However, the optimal type and intensity of the preparative regimen remains unclear. We evaluated the toxicity and outcomes of a conditioning regimen consisting of melphalan 140 mg/m2 (day −8), thiotepa 10 mg/kg (day −7), fludarabine 160 mg/m2 over 4 days (days −6 to −3), and rabbit ATG 1.25 mg/kg (day −4) and 1.75 mg/kg (day −3) (FMT). Forty-seven patients with advanced hematologic malignancies with a median age of 23 years (30 adults and 17 children) were treated. Sixty percent of patients were in remission at transplant. Ninety-one percent of the patients engrafted neutrophils after a median of 22 days, and all but one of the patients achieving donor engraftment had hematopoietic recovery with 100% cord blood-derived cells. Grade 3 gastrointestinal toxicity was the major non-hematopoietic toxicity occurring in 32% of patients. Cumulative incidence of day-100 grade II-IV aGVHD and cGVHD were 53% and 34%, respectively, and non-relapse mortality at day 100 and 2 years was 11% and 40%. Two-year disease-free and overall survival rates were 31% and 44%, respectively. These results suggest that FMT is a feasible conditioning regimen for patients undergoing CBT.
Keywords: Unrelated cord blood transplantation, reduced-intensity conditioning, fludarabine, melphalan, thiotepa
Introduction
Unrelated cord blood transplantation (CBT) is an alternative treatment option for patients who lack a matched sibling or unrelated donor.1-3 Better outcomes have been reported in children with disease–free survival of up to 70% while outcomes in adult patients are less favorable, primarily due to the limited number of progenitor cells per kilogram available in an umbilical cord blood (CB) unit.3-6 The use of double CB units for transplants has been reported to improve engraftment and potentially improve outcomes.7 However, the optimal conditioning regimen for cord blood transplantation remains unclear. Non-total body irradiation containing regimens may decrease treatment-related mortality (TRM).8,9 Recently, a regimen consisting of busulfan in combination with fludarabine and thiotepa has been described for CBT patiens.10 Here we report our experience with melphalan, fludarabine, thiotepa and anti thymocyte globulin (ATG) for patients with advanced hematologic malignancies receiving single or double CB transplants.
MATERIALS AND METHODS
Patients
This is a retrospective study of 47 consecutive patients with advanced hematologic malignancies who underwent CBT at 3 institutions between August 2003 and May 2008. Thirty-seven patients were treated at the University of Texas MD Anderson Cancer Center (MDACC) on two clinical trials, one using unmanipulated and another one investigating ex-vivo CB expansion, while 10 patients were treated at Hospital Israelita, Sao Paolo, Brazil and Instituto de Cancerologia Las Americas, Medellin, Colombia. Patients were eligible to receive CBT if they were less than 60 years old, met standard accepted criteria for advanced/high-risk hematologic malignancies and had no matched sibling or unrelated donors available (8 of 8 or 7 of 8 HLA-A, -B, -C, -DRB1). One or two CB units were identified (at least 4/6 HLA-A, -B and -DRB1 match between the unit and the recipient and between the 2 units in double CBT, with at least 2x107 total nucleated cell (TNC)/kg). Thirty patients received two CB units while 17 only one unit (7 treated at MDACC and 10 at the outside institutions). Prior to year 2005, a small number of patients were treated with a 3/6 HLA match CB unit and a TNC dose of <2x107/kg. All patients provided written informed consent approved by each Institutional Review Board (IRB) according to the Declaration of Helsinki. An IRB approved data review protocol with a waiver of informed consent was obtained for this retrospective study.
Cord Blood Unit HLA typing, Selection and Processing
HLA typing of the units was done by low/intermediate resolution for HLA-A,-B and high resolution for HLA-DRB1. All selected CB units tested negative for human immunodeficiency virus type I, hepatitis B, C and HTLV-1 viruses. Thawing and washing of the CB units was performed according to the method previously described by Rubinstein et al.11
Conditioning Regimen, Graft-versus-Host Disease Prophylaxis and Supportive Care
The conditioning regimen consisted of melphalan 140 mg/m2 on day −8, thiotepa 10 mg/kg on day −7, fludarabine 160 mg/m2 in divided doses given on days −6, −5, −4 and −3 and rabbit ATG 1.25 mg/kg on day −4 and 1.75 mg/kg on day −3. In addition, patients received tacrolimus or cyclosporine plus methotrexate or tacrolimus plus mycophenolate mofetil (MMF) for GVHD prophylaxis (Table 1). Methotrexate 5 mg/m2/day was given on days −1, 3 and 6. The MMF was continued until day 40, and tacrolimus or cyclosporine until 6 months post transplant and then tapered.
Table 1.
Patients’ Characteristics
| Overall (N=47) | % | Adult (N=30) | % | Pedi (N=17) | % | |
|---|---|---|---|---|---|---|
| Age (median, range) | 23 (1-60) | 35 (19-60) | 6 (1-17) | |||
| Location MDACC | 37 | 79 | 28 | 93 | 9 | 53 |
| Other | 10 | 21 | 2 | 7 | 8 | 47 |
| Gender F/M | 13/34 | 28/72 | 10/20 | 3/14 | ||
| Diagnosis AML/MDS | 9 | 19 | 7 | 23 | 2 | 12 |
| ALL | 21 | 45 | 9 | 30 | 12 | 71 |
| CML | 3 | 6 | 0 | 0 | 3 | 18 |
| CLL | 1 | 2 | 1 | 3 | 0 | 0 |
| NHL | 3 | 6 | 3 | 10 | 0 | 0 |
| Hodgkin's | 10 | 21 | 10 | 33 | 0 | |
| # prior chemotherapy regimens ≤4 | 22 | 47 | 14 | 47 | 8 | 47 |
| >4 | 23 | 49 | 15 | 50 | 8 | 47 |
| Disease status at transplant CR/CRp | 27 | 57 | 13 | 43 | 14 | 82 |
| Other | 20 | 43 | 17 | 57 | 3 | 18 |
| GVHD prophylaxis Tacro+MMF | 16 | 34 | 12 | 40 | 4 | 24 |
| Tacro+MTX | 23 | 49 | 18 | 60 | 5 | 29 |
| Tacro alone | 1 | 2 | 0 | 0 | 1 | 6 |
| CSA+MTX | 7 | 15 | 0 | 0 | 7 | 41 |
| Cord type Single | 17 | 36 | 4 | 13 | 13 | 76 |
| Double | 30 | 64 | 26 | 87 | 4 | 24 |
| Single Expanded | 7 | 15 | 2 | 7 | 5 | 29 |
| Unmanipulated | 10 | 21 | 2 | 7 | 8 | 47 |
| Double Expanded | 12 | 26 | 11 | 37 | 1 | 6 |
| Unmanipulated | 18 | 38 | 15 | 50 | 3 | 18 |
| Number of HLA mm (of 6) 0 | 1 | 2 | 0 | 0 | 1 | 6 |
| 1 | 8 | 17 | 5 | 17 | 3 | 18 |
| 2 | 31 | 66 | 18 | 60 | 13 | 76 |
| 3 | 4 | 9 | 4 | 13 | 0 | 0 |
| Median | 95% CI | Median | 95% CI | Median | 95% CI | |
| Total TNC (×10e8/kg) | 0.36 | 0.1-1.5 | 0.35 | 0.1-0.55 | 0.53 | 0.15-1.5 |
| Total CD34 (×10e6/kg) | 0.14 | 0.01-1.3 | 0.1 | 0.01-0.45 | 0.2 | 0.04-1.3 |
Figure legend: MDACC-MD Anderson Cancer Center; F-female, M-male; AML-acute myelogenous leukemia, MDS-myelodysplastic syndromes; ALL-acute lymphoblastic leukemia; CML-chronic myelogenous leukemia; CLL-chronic lymhocytic leukemia; NHL-non-Hodgkin's lymphoma; CR-complete remission; CRp-complete remission without platelet recovery; Tacro-tacrolimus; MMF-myecophenolate; MTX-methotrexate; CSA-cyclosporine; TNC-total nucleated cells.
All patients received anti-bacterial prophylaxis with levofloxacin, anti-viral prophylaxis with foscarnet or gancyclovir followed by valacyclovir, and anti-fungal prophylaxis with voriconazole or caspofungin. Pneumocystis carinii prophylaxis was employed with pentamidine, atovaquone or trimethoprim-sulfamethoxazole.
Definitions
Advanced disease was defined as not in first remission at the time o transplant. Engraftment was defined as achieving an absolute neutrophil count (ANC) greater than 0.5 K/UL for more than 3 consecutive days before day 42, with donor derived cells detected by DNA microsatellite analysis. FISH for Y chromosome in sex mismatched transplants was accepted as an alternative for centers in which polymerase chain reaction (PCR) was not readily available. Engraftment after day 42 with donor hematopoiesis was considered “delayed”. Platelet recovery was defined as the first day on which the platelet count was greater than 20 K/UL unsupported by platelet transfusions for 7 days. Primary graft failure was defined as failure to achieve an ANC > 0.5 K/UL by day +42, and secondary graft failure as sustained graft loss (fall of ANC to < 0.5 K/UL) for > 5 days after initial engraftment. Acute GVHD and chronic GVHD were graded according to the previously described criteria.12,13 Other toxicity was scored using NCI criteria.14
Statistical Analysis
The primary endpoint was engraftment, whereas toxicity, TRM, overall survival (OS), and progression-free survival (PFS) were secondary endpoints. Actuarial estimate of OS and PFS were calculated using the Kaplan-Meier method.15 The rate of TRM, acute and chronic GVHD, and engraftment were estimated using the cumulative incidence method to account for competing events.16 Chi square and Fisher's exacts test were used to assess the association between categorical variables17, Cox proportional hazard regression methodology was used to evaluate prognostic factors for TRM, OS and PFS in adult patients.18 Risk factors evaluated included age (dichotomized at the median), number of prior chemotherapy regimens (>4 vs. ≤4), disease status at transplant (CR vs. other), the use of MMF for GVHD prophylaxis, cord type (single vs. double), expansion in double cords, TNC cell dose infused (categorized at the quartiles) and CD34+ cell dose infused (categorized at the quartiles). Multivariate analysis evaluating the independent prognostic factors was not possible due to sample size limitations.
Statistical significance was defined at the 0.05 level. Statistical analysis was performed using STATA 9.0 (SataCorp. 2005. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP) and R version 2.10.1 (http://www.R-project.org).
RESULTS
Patients and Cord Blood Unit Characteristics
Patient characteristics are presented in Table 1. A total of 47 patients were treated at 3 institutions including MDACC (n=37), Instituto de Cancerologia Las Americas, Medellin, Colombia (n=7) and Hospital Israelita, Sao Paolo, Brazil (n=3). Thirty patients were adults, 17 children. Median age was 23 years (range 1-60) and median patient weight was 68 kg (range 11-101 kg). Patients were heavily pretreated with a median number of prior systemic chemotherapy courses of 5 (range 1-20). Cytogenetics were poor-risk in 12 of 30 patients (40%) with acute leukemia. Of the 30 patients with acute leukemia, 9 were in CR1 (30%), 17 were in CR ≥ 2 (57%) and 4 were not in CR. Fourteen patients had lymphoma/CLL, 10 Hodgkin's disease, 3 Non-Hodgkin's lymphoma, 1 CLL, all but one not in complete remission at the time of transplant. Eight of 10 patients with Hodgkin's lymphoma had prior autologous stem cell transplant.
A total of 77 CB units were infused; 30 patients received double CBT and 17 patients single CBT. Twenty-eight patients received unmanipulated graft while 19 patients received expanded units as part of 2 clinical protocols, an ex-vivo expansion study which involved a copper chelator (Gamida cell trial)19 or mesenchymal stromal cells20. The great majority of units were matched with the patient at 4/6 HLA antigens (66%) using antigenic level testing for class I and high-resolution molecular testing for class II. All but one pediatric patient received units that were <6/6 HLA-match (Table 1).
The median number of TNC and CD34+ cell dose infused (sum of CB 1 and 2 if double units infused) was 3.6x107/kg (range 1-14.7) and 1.4x105/kg (range 0.1-13.4), respectively. Data for CD34+ cell numbers was available in 45/47 treated patients.
Engraftment
Two patients died early (day +26 and day +42) due to sepsis/worsening pneumonia and progressive disease without engraftment. Forty-three patients (91%) engrafted with donor-derived hematopoiesis, all by day 42 except one who engrafted after 45 days. The cumulative incidence of engraftment by day +50 was 90% (95% CI 80-100) in adults, and 94% (95% CI 84-100) in children. Engraftment of donor derived cells was documented by PCR in all but 4 patients, where confirmation was documented by FISH for Y chromosome. Thirty-seven patients treated at MDACC had detailed data on chimerism. Twenty-nine of these patients had double cord blood transplants and 8 single unit transplants. Eighteen patients received unmanipulated cord blood units while 19 patients had one cord blood unit expanded as described above. Of 37 patients, 2 had early death and 1 had primary graft failure. Thirty-three of 34 remaining patients engrafted with 100% donor cells (one had mixed chimerism), in 21 (62%) patients the chimerism was derived from one unit while in 13 patients was a mixed chimerism between the two units. First unit infused ultimately engrafted in 25 (74%) patients, second unit infused engrafted in 7 patients, while in 2 patients, at the last follow-up, the winning unit was not yet clear. Donor-specific anti-HLA antibodies as previously reported by us19 could not be implicated in graft rejection nor in predominance of one unit versus the other due to the fact that and only a subgroup of patients had HLA antibody testing performed. Of the 7 patients who engrafted with the second unit only 3 patients were tested, 2 did not have HLA antibodies while 1 had anti-HLA antibodies against B49 locus from the first cord blood unit and engrafted with the second unit infused.
Median time to neutrophil engraftment was 22 days overall, 21 days in adults and 22 days in pediatric patients. Among patients who engrafted, quartiles analysis showed a significant correlation between cell dose and time to engraftment. Considering delayed engraftment as neutrophil recovery occurring after 28 days, there was a significantly higher proportion of patients with delayed engraftment among those who received CD34+ cell doses falling within (45%) the first quartile compared to those with doses above the first quartile (45% vs. 13%, p=0.04). A similar association was observed for TNC (58% vs. 10%, p=0.002). The cumulative incidence of platelet engraftment with the competing risk of death was 68%. Platelet engraftment occurred in 32 patients after a median time of 35 days (range 25-157).
Chimerism data on day 30 post transplant for the 37 patients treated at MDACC showed that out of the 8 patients who received a single CBT, 7 engrafted with 100% donor myeloid and T-cells while for 1 patient the chimerism was not available; for the 29 patients who had a double CBT, the T-cell chimerism was 100% (for one of the two cords) in 18 patients, mixed T-cell chimerism between the two cords in 6 patients, suboptimal or not available in 5 patients. Myeloid chimerism for these patients showed a full (100%) myeloid chimerism in 16 patients, mixed chimersim between the two cords in 9 patients, and was suboptimal or not available in 4 patients. No tested patient had a mixed chimerism between the donor and recipient for either myeloid or T-cells.
Graft-versus-host Disease
GVHD was a major cause of morbidity and mortality in these patients. GVHD prophylaxis was employed with a combination of immunosuppressants, all including calcineurin inhibitor-based. Forty patients (85%) received tacrolimus for GVHD prophylaxis, 23 patients (49%) in combination with methotrexate and 16 (34%) in combination with MMF (Table 1). Cumulative incidence of grade II-IV aGVHD and grade III-IV aGVHD within 100 days was 40% and 13% respectively for the whole group, with no significant differences between adults and children (Table 2). All 6 patients with grade III-IV aGVHD died. GVHD prophylaxis for these patients consisted of tacrolimus and methotrexate (n=4) and tacrolimus and MMF (n=2). Cumulative incidence of cGVHD was 34% overall, higher in adults (43%) versus pediatric patients (18%) (p=0.05) (Table 2). Eighty percent of the cGVHD cases were extensive.
Table 2.
Outcomes of 47 patients treated with cord blood transplantation who received fludarabine, melphalan, thiotepa and ATG conditioning.
| Overall (N=47) | Adult (N=30) | Pedi (N=17) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Engrafted, n (%) | 43 (91) | 27 (90) | 16 (94) | ||||||
| Days to ANC 500 (median, (range)) | 22 | (6-115) | 21 | (6-45) | 22 | (11-115) | |||
| Days to PLT 20,000 (median, (range)) | 35 | (25-157) | 35 | (27-134) | 34 | (25-157) | |||
| (%) | 95% CI | (%) | 95% CI | (%) | 95% CI | *HR | 95% CI | p | |
| aGVHD, %CI | |||||||||
| II-IV | 40% | 28-57 | 36% | 19-55 | 47% | 28-78 | 0.8 | 0.3-1.9 | 0.6 |
| III-IV | 13% | 6-28 | 14% | 6-35 | 12% | 3-43 | 1.2 | 0.2-6.9 | 0.8 |
| cGVHD, %CI | 34% | 22-51 | 43% | 28-66 | 18% | 6-51 | 3.6 | 1.0-1.3 | 0.05 |
| NRM, %CI | |||||||||
| 100 days | 11% | 5-24 | 13% | 5-33 | 6% | 1-39 | 2.2 | 0.2-20 | 0.5 |
| 1 year | 28% | 18-44 | 30% | 17-52 | 25% | 11-60 | 1.3 | 0.4-4.3 | 0.6 |
| 2 years | 40% | 28-58 | 43% | 29-65 | 33% | 16-69 | 1.8 | 0.6-5.6 | 0.3 |
| Last f/up | 40% | 28-58 | 43% | 29-65 | 33% | 16-69 | 1.5 | 0.5-4.3 | 0.4 |
| Disease progression, %CI | |||||||||
| 100 days | 0% | NA | 0% | NA | 0% | NA | NA | NA | NA |
| 1 year | 20% | 11-35 | 20% | 10-41 | 18% | 6-49 | 1.2 | 0.3-4.9 | 0.8 |
| 2 years | 24% | 15-41 | 28% | 15-50 | 18% | 6-49 | 1.7 | 0.5-6.6 | 0.4 |
| Last f/up | 32% | 19-55 | 40% | 23-72 | 18% | 6-49 | 2 | 0.5-7.6 | 0.3 |
| Survival, %KM | |||||||||
| 100 days | 85% | 71-93 | 83% | 64-93 | 88% | 61-97 | 1.4 | 0.3-7.2 | 0.7 |
| 1 year | 59% | 43-71 | 60% | 40-75 | 55% | 28-75 | 0.9 | 0.4-2.5 | 0.9 |
| 2 years | 44% | 29-58 | 38% | 20-55 | 55% | 28-75 | 1.5 | 0.6-3.5 | 0.4 |
| last f/up | 30% | 16-46 | 19% | 5-39 | 47% | 21-69 | 1.6 | 0.7-3.7 | 0.2 |
| PFS, %KM | |||||||||
| 100 days | 85% | 71-93 | 83% | 64-93 | 88% | 61-97 | 1.4 | 0.3-7.2 | 0.7 |
| 1 year | 48% | 33-61 | 47% | 28-63 | 50% | 24-72 | 1.2 | 0.5-2.8 | 0.7 |
| 2 years | 31% | 17-45 | 26% | 12-42 | 43% | 19-66 | 1.7 | 0.7-3.8 | 0.2 |
| Last f/up | 23% | 9-41 | 13% | 1-38 | 43% | 19-66 | 1.7 | 0.8-3.6 | 0.2 |
Figure legend: ANC-absolute neutrophil count; PLT-platelets; aGVHD- acute graft-versus-host-disease; cGVHD-chronic graft-versus-host-disease; NRM-non-relapse mortality; OS-overall survival; PFS-progression-free survival. % CI cumulative incidence, % KM Kaplan-Meier.
Toxicity, Non-relapse Mortality and Causes of Death
Adverse effects are described for the 37 patients treated at MDACC, for whom detailed toxicity data was available. The major non-hematopoietic toxicity was grade 3 gastrointestinal (stomatitis, nausea, vomiting, and diarrhea) which occurred in 12 patients (32%). Three patients developed altered mental status, one secondary to steroids which resolved, while 2 patients had seizures in the setting of infections/hypoxia. These two patients were treated temporarily with plasma exchange for a presumptive diagnosis of thrombotic microangiopathy. Although suspected and treated, thrombotic microangiopathy could not be confirmed in any of these patients.
One patient developed a transient decrease in ejection fraction from 50-55% pre-transplant to 30-35% on day 55 after transplant.
Liver toxicity (grade 3 elevation in ALT/AST or hyperbilirubinemia) occurred in 3 patients but this was not considered regimen-related; this was caused by disseminated mycobacterium tuberculosis in one patient, disseminated adenovirus infection in another patient, and liver involvement by disease (CLL/SLL) in one other case. Acute pancreatitis occurred in one patient, and resolved with conservative management. There were no cases of veno-occlusive disease (VOD).
Grade 3 or greater renal toxicity occurred in 2 patients; in one patient this was a terminal event following a disseminated infection (as above) and another patient had acute renal failure on day +12 post transplant, for which he underwent temporary dialysis followed by normalization of creatinine and discontinuation of hemodialysis before the hospital discharge.
Causes of death included infection in 12 patients (25.5%), leukemia relapse in 11 patients (23.4%), graft failure (2 patients), GVHD (2 patients). One patient died of a bleeding complication after an endoscopic procedure performed to confirm. Infections were the major cause of non-relapse mortality and occurred in all patients evaluated. Of 37 treated patients, 16 had CMV reactivation (43%), 9 pts had dysuria/hematuria/bladder pain (24.3%), in 8 associated with BK viruria. Pneumonia was the most common infection and occurred in 22/37 patients treated at MDACC (total of 38 episodes). Median time to occurrence was 144 days, 16 (40%) occurred before day 100. Organisms were viral 37%, bacterial 24%, fungal 18%. Overall, cumulative incidence of 100-day and 1 year TRM were 11% and 28%, respectively (Table 2). None of the prognostic factors evaluated were significantly associated with the rate of TRM among adult patients.
Relapse and Survival
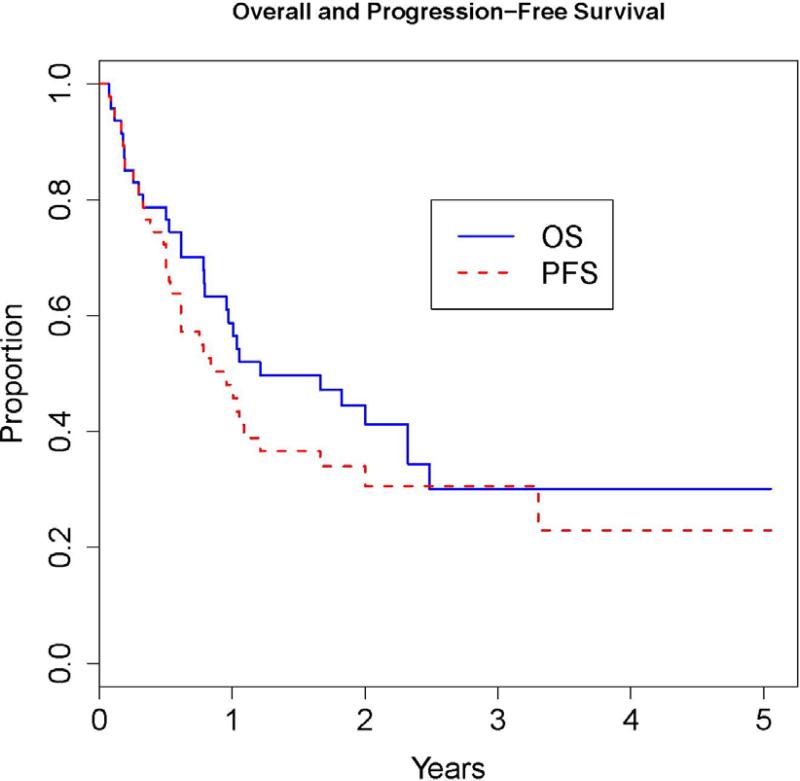
The median follow-up among survivors in the entire cohort was 2.3 years (range 0.5-5.1). Overall, 12 patients progressed/relapsed at a median of 7 months (range 4-40 months), 6 of whom were not in remission at the time of transplant. Actuarial one-year and two-year overall survival for the whole group was 59% and 44%, and progression-free survival 48% and 31%, respectively (Table 2, Figure 1). Evaluation of prognostic factors among adult patients showed a significantly higher mortality rate in patients older than 35 years (HR=3.4, P=0.02). There was also a trend for lower mortality rate for patients who received CD34+ cell dose falling within the highest quartile [>10.16 e6/Kg; (HR=0.2, P=0.06)] or a TNC dose above the median [>0.35 e8/Kg); (HR=0.4, P=0.09)]. The rate of progression-free survival was significantly lower in patients who received CD34+ cell doses within the highest quartile (HR=0.2, P=0.03); and marginally significant for those who received TNC dose above the median (HR=0.4, P=0.05). Patients in remission at transplant had 69% OS and 54% PFS at 1 year; and 40% and 31% at 2 years respectively. In contrast, patients not in remission had 53 % OS and 41% PFS at 1 year; and 40% and 22% at 2 years, respectively. For both OS and PFS, there was no evidence of a relationship between these outcomes and the number of prior chemotherapies (HR=0.8, p=0.6) and (HR=0.9 p=0.8), respectively.
Figure 1.
Overall survival (OS) and progression-free survival (PFS) for the whole group of patients (N=47).
DISCUSSION
Achievement of durable engraftment remains a major problem with cord blood transplantation, related to the relatively low hematopoietic cell dose and the presence of HLA disparities between donors and recipients. In this study we evaluated engraftment, toxicity and outcomes of cord blood transplant recipients treated with fludarabine, melphalan, thiotepa and ATG conditioning. We have previously reported on this regimen in patients treated with T-cell depleted haploidentical stem-cell transplantation.22 As previously shown, this regimen was well tolerated especially by younger patients, and was associated with high rate of engraftment and relatively low treatment-related mortality. The major toxicity involved gastro-intestinal adverse effects. Limited or reversible renal, cardiovascular and liver toxicity (without VOD) observed with this regimen were very similar to those we reported in haploidentical transplantation.22
Engraftment in cord blood transplantation with this regimen was better (overall 94% including one patient with delayed engraftment) as compared with our previous experience with fludarabine 160mg/m2 with either 13Gy TBI or melphalan 180 mg/m2 (without thiotepa) regimens (27% graft failure).23 Moreover, toxicity especially with TBI-based regimen was unacceptably high with NRM of 40% at day 100 and 50% at 1 year.23
In an attempt to decrease TRM and increase engraftment rate we treated a small number of patients with fludarabine 160mg/m2, melphalan 140 mg/m2 and ATG. A third of these patients failed to engraft (data not published). Similar experience was reported by Narimatsu et al.24 This group treated 10 patients with a conditioning regimen consisting of fludarabine, melphalan 140-180mg/m2 and ATG. Only 5/10 pts engrafted and 5 died before day 100 due to TRM.24
We also investigated a myeloablative busulfan-based regimen and treated 11 patients with myeloid malignancies and double UCBT (fludarabine 160mg/m2 and i.v. busulfan 520mg/m2 total dose divided in 4 daily doses, and ATG) (BuFlu). Five of 11 patients failed to engraft. Similar results were reported by Horwitz et al.25 This group treated 10 UCBT patients with the same drugs without ATG.25 Eight of 10 patients rejected the graft and the study was closed.23 This group concluded that i.v. BuFlu in the dose and schedule studies does not provide enough immunosupression to allow sustained engraftment of UCB stem cells.25
Our experience described in this report shows that improved engraftment was achieved with the addition of thiotepa (10 mg/kg) to fludarabine (160 mg/m2) and melphalan 140mg/m2. Similar rates of engraftment were reported recently by Sanz et al. with a regimen consisting of fludarabine, busulfan and thiotepa.10 Taken together, these reports suggest that thiotepa could provide more immunosupression to either standard fludarabine melphalan or fludarabine and busulfan regimens, leading to improved engraftment in these patients.
Treatment failures in this study were mostly related to infections and relapse. Persistent immune deficiency 26,27 may predispose to infectious complications more than a year after transplant and could contribute to continued occurrence of NRM in these patients.
The role and amount of ATG in the preparative regimen remains debatable. It has been previously reported that ATG could increase the risk of hemorrhagic cystitis, Epstein-Barr virus (EBV) related post-transplant lymphoproliferative disorder in cord blood transplant recipients.28,29 The use of alternative strategies for immunosupression may favor a faster immunologic reconstitution and a lower incidence of infectious complications. The incidence of EBV reactivation in this study was modest (only 2 patients).
In conclusion, the preparative regimen of fludarabine, melphalan, thiotepa and ATG provided reliable engraftment for unrelated cord blood transplantation. Future studies should be directed towards increasing the speed of engraftment, decreasing rate of infectious complications and GVHD. Gastrointestinal effects were the major regimen-related toxicity and we are exploring dose modifications of melphalan and thiotepa to reduce these effects.
Footnotes
Authorship
S.O.C. contributed to study design, collected the data and wrote the paper; R.M.S. performed statistical analysis and helped write the paper; R.B. performed the statistical analysis; N.H. and A.K.A contributed to patient accrual, data collection, reviewed and approved the manuscript; M.F.V. performed the HLA antibody testing, reviewed and approved the manuscript; G.R., J.M. contributed to data collection, reviewed and approved the manuscript; D.P., L.L.W., K.W.C., D.C., R.B.J., P.K., C.H., Y.N., contributed to patient accrual, reviewed and approved the manuscript; E.J.S., R.E.C. and M.L. contributed to patient accrual, study design, data collection, revised and approved the manuscript.
Conflict of interest: N/A
Conflict of interest
The authors have no conflict on interest to declare.
References
- 1.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 5.Long GD, Laughlin M, Madan B, et al. Unrelated umbilical cord blood transplantation in adult patients. Biol Blood Marrow Transplant. 2003;9:772–780. doi: 10.1016/j.bbmt.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 7.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 8.Giralt S, Thall PF, I. Khouri I, et al. Melphalan and purine analog–containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 9.Slavin S, Nagler A, Naperstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreductionfor the treatment of malignant and nonmalignant diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 10.Sanz J, Sanz MA, Saavedra S, et al. Cord blood transplantation from unrelated donors in adults with high risk acute myeloid Leukemia. Biol Blood Marrow Transplant. 2010;16:86–94. doi: 10.1016/j.bbmt.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Martin P, Klingmann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 13.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 14.Common Toxicity Criteria. Version 3.0. Available at: http://ctep.info.nih.gov/reporting/ctc.html.
- 15.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 17.Agresti A. A Survey of Exact Inference for Contingency Tables. Stat Sci. 1992;7:131–53. [Google Scholar]
- 18.Cox DR. Regression models and life tables [with discussion]. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 19.de Lima M, McMannis J, Gee A, et al. Transplantation on ex vivo expanded cord blood cells using copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lima M, Robinson S, McMannis J, et al. Mesenchymal stem cell (MSC)based cord blood (CB) expansion (Exp) leads to rapid engraftment of platelets and neutrophils. Blood. 2010;116:362a. [Google Scholar]
- 21.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45:429–436. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lekakis L, Giralt S, Couriel D, et al. Phase II study of unrelated cord blood transplantation for adults with high-risk hematologic malignancies. Bone Marrow Transplant. 2006;38:421–426. doi: 10.1038/sj.bmt.1705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narimatsu H, Watanabe M, Kohno A, et al. High incidence of graft failure in unrelated cord blood transplantation using reduced-intensity preparative regimen consisting of fludarabine and melphalan. Bone Marrow Transplant. 2008;41:753–756. doi: 10.1038/sj.bmt.1705978. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz ME, Morris A, Gasparetto C, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant. 2008;14:591–594. doi: 10.1016/j.bbmt.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komanduri K, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciurea SO, Mulanovich V, Jiang Y, et al. Lymphocyte recovery predicts outcomes in cord blood and T-cell depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2010 Nov 30; doi: 10.1016/j.bbmt.2010.11.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunstein CG, Weisdorf DJ, DeFor T, et al. Increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballen KK, Cutler C, Yeap BY, et al. Donor-deriver second malignancies after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:1025–1031. doi: 10.1016/j.bbmt.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]