Table 3.
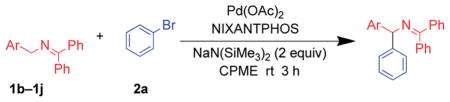
| |||
|---|---|---|---|
| Entry | Ketimine | Pd(OAc)2/NIXANTPHOS (mol%) | Yield (product) |
| 1 |
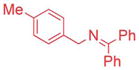
|
5/7.5 | 89 (3ac) |
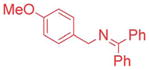
| 2 |
|
10/20 | 71c (3ca) |
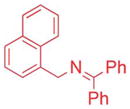
| 3 |
|
5/7.5 | 71d (3af) |
| 4 |
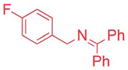
|
2.5/3.75 | 83 (3ah) |
| 5 |
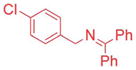
|
2.5/3.75 | 89 (3ag) |
| 6 |

|
2.5/3.75 | 91 (3ga) |
| 7 |
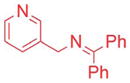
|
5/7.5 | 87 (3aj) |
| 8 |
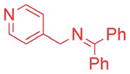
|
10/20 | 90e (3ia) |
| 9 |

|
10/20 | 60f (3ja) |
Reactions conducted on a 0.1 mmol scale using 2 equiv. of imine, 2 equiv. of NaN(SiMe3)2, and 1 equiv. of bromobenzene at 0.1 M. Base was added portionwise at 0.1 mL per 30 min.
Isolated yield after chromatographic purification.
3 equiv. of LiO–tBu 80 °C in 3 mL THF for 12 h.
3 equiv of 1d and 3 equiv. of NaN(SiMe3)2.
2 equiv. LiN(SiMe3)2, 50 °C.
50 °C.
