Table 5.
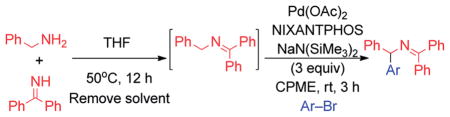
| |||
|---|---|---|---|
| Entry | Ar–Br | Pd(OAc)2 (mol%)/NIXANTPHOS (mol%) | Yield (%) |
| 1 |
|
5/7.5 | 90 (3ag) |
| 2 |
|
5/7.5 | 92 (3ab) |
| 3 |
|
5/7.5 | 63 (3ae) |
Reactions conducted on a 0.1 mmol scale using 3 equiv. of benzyl amine, 3 equiv. of benzophenone imine, 3 equiv. of NaN(SiMe3)2, and 1 equiv. of aryl bromide at 0.1 M. Base was added portionwise at 0.1 mL per 30 min.
Isolated yield after chromatographic purification.
