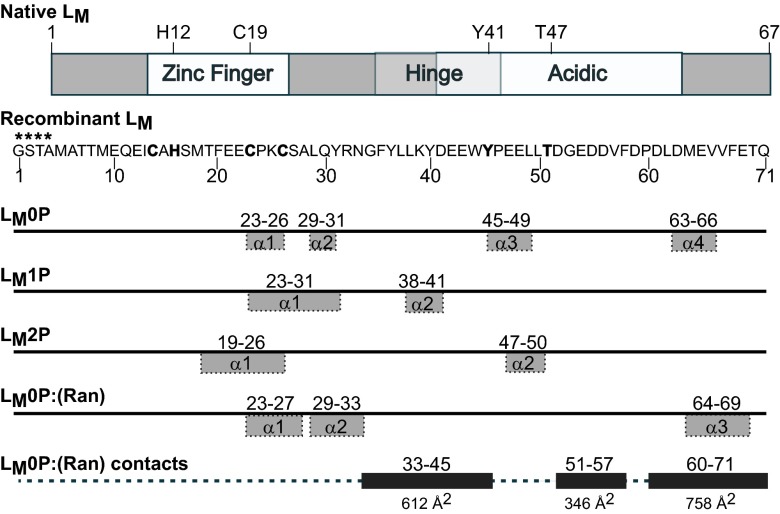
Fig. 1.
LM schematics. Protein map of native LM shows motifs and the Y41, T47 phosphorylation sites; the sequence of LM as determined by NMR is 4 aa longer (****) than the native protein at the NH2 end. NMR-determined α-helix motifs were defined by TALOS+ for respective structures (Fig. S5A). The remainder of each protein is random coil. The inclusive residue segments that shift on binding with Ran are indicated with the extent of their contact surfaces (Å2) in the docked (Ran):LM0P structure.