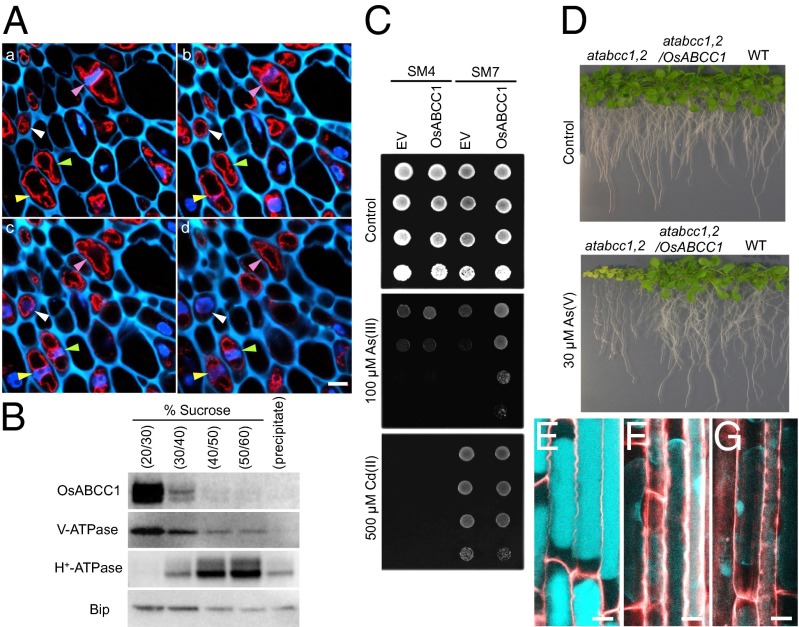
Fig. 4.
Subcellular localization of OsABCC1 and As resistance mediated by OsABCC1. (A) Immunostaining analysis of subcellular localization of OsABCC1. Double staining with OsABCC1 antibody and DAPI was performed in node I of rice at the flowering stage. OsABCC1 antibody (red) and DAPI (nucleus; blue) fluorescence and cell wall autofluorescence (cyan) at four consecutive optical sections of 2.5-µm intervals (a–d) of the same sample are shown. Different colored arrowheads indicate the position of nuclei in each cell. (Scale bar, 5 µm.) (B) Immunoblot analysis of OsABCC1 localization. Microsomes extracted from rice roots were fractionated by sucrose density gradient. Polyclonal antibodies of anti-OsABCC1, anti–V-ATPase (tonoplast marker), anti–H+-ATPase (plasma membrane marker), and BiP (ER marker) were used. (C) Phytochelatin-dependent As resistance mediated by OsABCC1. Yeast strains SM4 and SM7 transformed with empty vector (EV) or OsABCC1 were cultured in half-strength SD agar plates with or without As(III) or Cd(II) at 30 °C for 3 d. (D) Recovery of As resistance in the Arabidopsis atabcc1 atabcc2 double mutant (atabcc1,2) by the introduction of OsABCC1. Wild-type (WT), atabcc1,2, and atabcc1,2 Arabidopsis lines transformed with OsABCC1 were grown on half-strength MS agar media supplemented with or without 30 µM As (V) for 3 wk. (E–G) Subcellular localization of thiol compounds. Seedlings (3-d-old) of WT rice (E), the osabcc1-1 mutant (F), and the osabcc1-2 mutant (G) were exposed to 0.5 µM As(III) for 3 h and then the roots were stained with monobromobimane for thiol compounds (cyan) and propidium iodide for the cell wall (red). Plasmolysis was induced by placing roots in 8% mannitol. Merged images are shown. (Scale bar, 10 µm.)