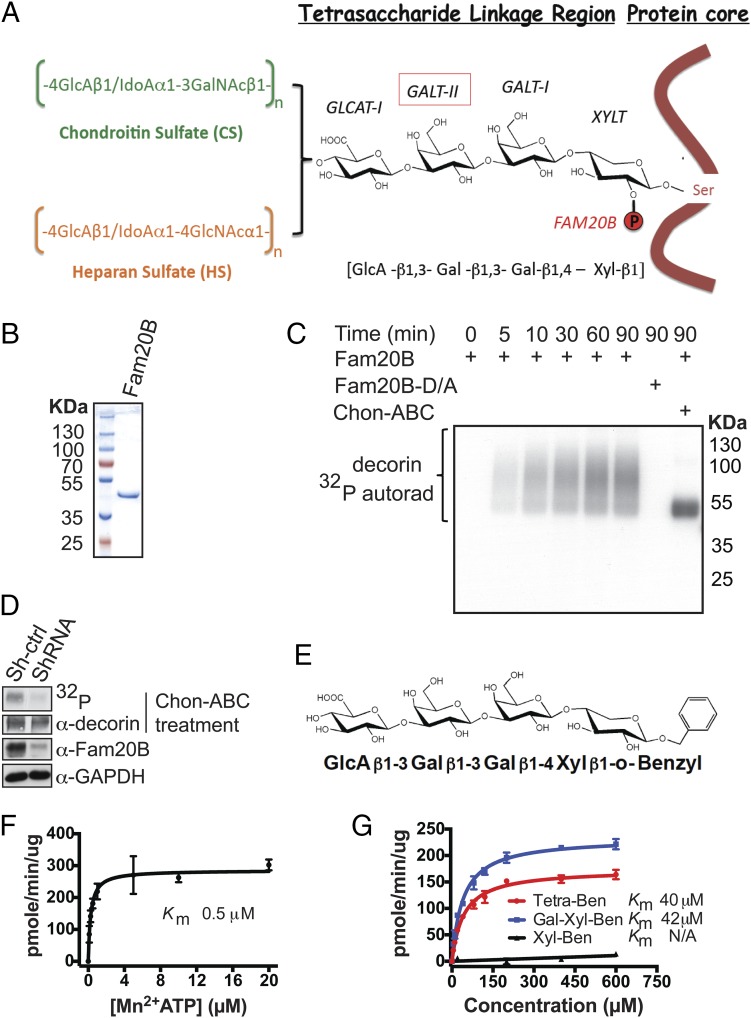
Fig. 1.
Xylosyl kinase Fam20B phosphorylates authentic proteoglycans. (A) Schematic representation of proteoglycan core proteins, glycosaminoglycan side chains, structure of the linkage tetrasaccharide, and biosynthetic enzymes involved. (B) Gel electrophoresis and Coomassie staining of recombinant Fam20B protein purified from insect cell conditioned medium. (C) Time-dependent incorporation of 32P from [γ-32P]ATP into decorin by Fam20B or Fam20B D309A (D/A) treated with or without chondroitinase ABC (Chon-ABC). Reaction products were analyzed by gel electrophoresis and autoradiography (autorad). (D) Lentiviral ShRNA mediated knockdown of Fam20B in MRC-5 cells and [32P]orthophosphate metabolic labeling. Fam20B knockdown efficiency was determined by immunoblotting of endogeneous Fam20B. The level of decorin phosphorylation in the control (Sh-ctrl) and Fam20B knockdown (ShRNA) cells were visualized by 32P autoradiography after decorin immunoprecipitation and gel electrophoresis. (E) Structure of synthetic Tetra-Ben as a model substrate for Fam20B. (F) Fam20B kinase reaction velocity versus [Mn2+]ATP concentration using Tetra-Ben as a substrate. (G) Fam20B kinase reaction velocities versus concentration of Tetra-Ben, Gal-Xyl-Ben, and Xyl-Ben artificial substrates. The reaction products for F and G were purified using SepPak C18 cartridges and the incorporated 32P radioactivity was quantified by scintillation counting. Data points were fitted by nonlinear regression of the Michaelis–Menten equation. Error bars are SD of three independent experiments.