Abstract
Objective
The objective of this study is to evaluate the impact of the HIV Infant Tracking System (HITSystem) for quality improvement of early infant diagnosis (EID) of HIV services.
Design and Setting
This observational pilot study compared 12 months of historical preintervention EID outcomes at one urban and one peri-urban government hospital in Kenya to 12 months of intervention data to assess retention and time throughout the EID cascade of care.
Participants
Mother–infant pairs enrolled in EID at participating hospitals before (n = 320) and during (n = 523) the HITSystem pilot were eligible to participate.
Intervention
The HITSystem utilizes Internet-based coordination of the multistep PCR cycle, automated alerts to trigger prompt action from providers and laboratory technicians, and text messaging to notify mothers when results are ready or additional action is needed.
Main outcome measures
The main outcome measures were retention throughout EID services, meeting time-sensitive targets and improving results turn-around time, and increasing early antiretroviral therapy (ART) initiation among HIV-infected infants.
Results
The HITSystem was associated with an increase in the proportion of HIV-exposed infants retained in EID care at 9 months postnatal (45.1–93.0% urban; 43.2–94.1% peri-urban), a decrease in turn-around times between sample collection, PCR results and notification of mothers in both settings, and a significant increase in the proportion of HIV-infected infants started on antiretroviral therapy at each hospital(14 vs. 100% urban; 64 vs. 100% peri-urban).
Conclusion
The HITSystem maximizes the use of easily accessible technology to improve the quality and efficiency of EID services in resource-limited settings.
Keywords: early infant diagnosis, HIV, HIV infant tracking system, HIV-exposed infants, improving laboratory performance, Kenya, mHealth, prevention of mother-to-child transmission, technology, text messaging to mobile phones
Introduction
Without early diagnosis of HIV and antiretroviral therapy (ART), over half of HIV-infected infants will die by 2 years of age [1,2]. Initiation of ART before 12 weeks of age reduces mortality among HIV-infected infants by 76% [3,4]. In 2008, the WHO recognized the significant public health impact of early infant diagnosis (EID) and treatment of HIV and issued guidelines advising practitioners to treat all HIV-infected infants under the age of 2 years immediately rather than waiting for immunologic or clinical criteria [5]. Inefficient EID systems fail to identify, retain and rapidly start HIV-infected infants on ART. This combined with poor uptake and social barriers contribute to low (<30%) treatment initiation among HIV-infected children globally [6,7].
In 2006, the Kenyan government’s National AIDS and Sexually Transmitted Infections Control Programme (NASCOP) established National Pediatric EID Guidelines [8] that specify key time-sensitive strategies to diagnose and manage HIV infection among HIV-exposed infants. In comparison to point-of-care antibody tests that only reflect the mother’s serological status, diagnosing infants requires more costly and complex molecular testing (HIV DNA PCR) that is typically conducted offsite at central laboratories. The Kenya Medical Research Institute (KEMRI) partnered with PEPFAR and others to develop the necessary infrastructure to provide HIV DNA PCR testing for maternally exposed infants. In 2008, Kenyan national policy was amended to include rapid initiation of ART for all children less than 2 years of age confirmed HIV-positive with PCR testing [9].
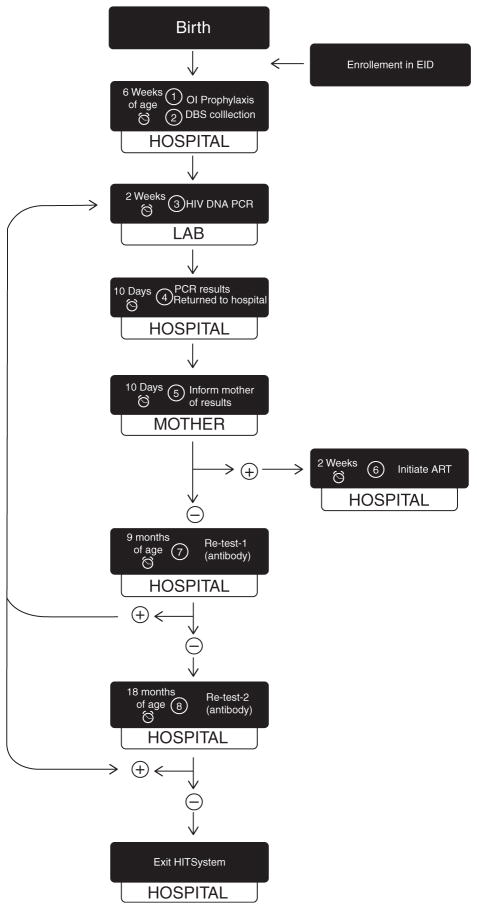
In 2010, the Kenyan EID system covered approximately 64% of health facilities nationally [10], however; these services are hampered by significant structural and operational barriers that contribute to late and sporadic testing of HIV-exposed infants [6,11,12]. For example, data collected between 2006 and 2008 from a district hospital in Kenya indicated late age at EID enrolment (68% after 2 months of age) and only 67% of those initially enrolled (156/213) ever had a dried blood spot (DBS) sample drawn for PCR testing [13]. Furthermore, greater than 30% of test results neither reach the intended health facility nor result in mother notification [9], leading to only 31% of Kenya’s 171 000 HIV-infected children receiving life-saving ART in 2012 [9,14]. EID data are maintained in multiple paper sources (e.g. registers, infant files) without a mechanism to track and prompt follow-up care or actively engage mothers in this process [6,12]. Consequently, prospective follow-up of infants is burdensome, and retention of HIV-exposed infants in care up to 18 months is low (15–35%) [6,13]. The absence of a systematic process for communication and accountability between providers, laboratories and mothers threatens retention at each point in the EID cascade of care (Fig. 1).
Fig. 1. HITSystem targets eight time-sensitive steps in the early infant diagnosis cascade of care.
Once enrolled, the infant’s date of birth is used to ensure that alerts for steps 1, 2, 7 and 8 occur by the designated infant age. Alerts for steps 3, 4, 5 and 6 are sent when steps have not been completed within the specified time interval, for example if an infant testing positive for HIV has not initiated ART within 2 weeks of mother notification. The alarm clock on the left of each box indicates the time-sensitive window that triggers electronic alerts requesting subsequent action to resolve them. The tab below each box indicates the location/responsible party for each step of the EID cascade of care. The loop on the left demarks the need for confirmatory PCR testing following all reactive antibody tests conducted at 9 and 18 months. Infants who test HIV-positive at any point in the cascade are initiated on ART. Infants confirmed HIV-uninfected at 18 months (or 6 weeks after breastfeeding cessation) complete and exit the HITSystem.
In this study, we assessed whether the HIV Infant Tracking System (HITSystem), an innovative online system utilizing algorithm-based computer alerts for EID and laboratory staff, and text messaging alerts to mothers, improves quality and efficiency of EID services in two Kenyan hospitals. The nongovernmental organization (NGO) Global Health Innovations (GHI) developed the HITSystem in 2010, in collaboration with the KEMRI with support from the NASCOP. We sought to determine whether the HITSystem improves retention throughout the EID cascade of care, meets time-sensitive targets and improves turn-around time for results, and increases early ART initiation among HIV-infected infants.
Materials and methods
Study setting, participants and design
The HITSystem intervention was piloted in two government hospitals: Pumwani Maternity Hospital in Nairobi and Kapsabet District Hospital in western Kenya. Inclusion criteria for hospitals included provision of prevention of mother-to-child transmission (PMTCT) services, EID, ART and maintaining infant medical files for all HIV-exposed infants enrolled in EID. Pumwani is the largest urban maternity hospital in Kenya, enrolling nearly 30 new HIV-infected mothers and their HIV-exposed infant per month. The peri-urban District Hospital in Kapsabet provides services to approximately 10–15 new mother–infant pairs monthly. These sites represent a high-volume urban hospital where mobile phone use is nearly universal and a lower-volume hospital in a peri-urban/rural setting with moderate mobile phone use and limited technological infrastructure. Standard of care in both hospitals included only paper-based record keeping in the HIV-exposed infant (HEI) Registries and relied on mothers’ initiative to return for test results and retesting.
We conducted an observational study with historical controls to evaluate the 12-month pilot of the HIT-System. As a system-level intervention, it was neither feasible nor acceptable to randomize participation at the individual level. This study did not require active recruitment because all mother/guardian–infant pairs presenting for EID care were eligible to utilize the HITSystem. Mobile phone ownership and literacy were not requirements for participation. Baseline characteristics were evaluated between study participants and historical controls. The control periods were April 2010 to March 2011 (urban) and June 2010 to May 2011 (peri-urban), and the intervention periods were April 2011 to March 2012 and June 2011 to May 2012. Twelve-month comparison periods were chosen to minimize seasonal variations that can affect hospital attendance.
HITSystem intervention
The HITSystem was designed to overcome the challenges of providing quality EID services in low-resource settings. This Internet-based program is accessible to team members at multiple locations (e.g. hospital and laboratory), captures and integrates clinical data on the basis of the Kenyan EID algorithm and uses the infant’s birth date to trigger electronic alerts when the EID cascade of care calls for time-sensitive actions (Fig. 1). Text messages are sent to mothers’ mobile phones when test results are available, medical treatment is indicated or routine retesting (9 and 18 months) is due. The system identifies receipt of text messages and absorbs all SMS costs. Messages do not relay sensitive information that could compromise confidentiality, but use either the field-tested message in Swahili, ‘Tafadhali mlete mtoto kwenye kliniki’ (please bring baby to the clinic), or a designated numeric code for nonliterate caretakers or those with heightened concern of stigma (e.g. shared mobile phone). Community health workers (CHWs) implement patient tracking procedures for mothers who do not respond to the text message or who do not own mobile phones.
Infants enrolled into the HITSystem are tracked until they are either conclusively determined to be HIV-uninfected at 18 months or 6 weeks after cessation of breast-feeding (whichever is later), and thus discharged from EID, determined to be HIV-infected, initiated on ART and linked to paediatric HIV care, transferred to another health facility or confirmed deceased. The prototype for the HITSystem was developed, vetted with stakeholders and systematically refined over a 2-year period to improve the feasibility, usability and outcomes of the system.
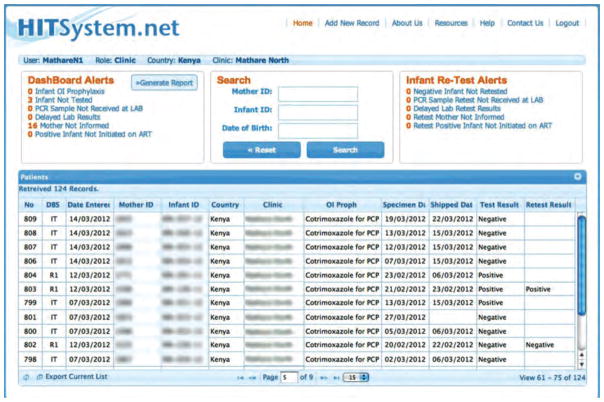
All automated alerts are linked to numeric identifiers and are updated daily on the HITSystem homepage for each site (Fig. 2). Alerts are only resolved once the specified action has been taken and recorded in the HITSystem by the EID provider (e.g. HIV-infected infant started on ART, or infant received 9-month retest). Kenyan and United States-based HITSystem managers can view alerts at each site and provide assistance and accountability for timely responses.
Fig. 2. HITSystem Dashboard Alerts.
This is the first screen that appears when a hospital logs into the HITSystem. The dashboard alerts in the red box are updated in real time and notify EID providers when time-sensitive interventions are overdue. For example, 16 mothers have not yet been informed of their infants’ PCR test results. This alert is triggered if the mother has not been notified within 2 weeks of the laboratory posting the PCR test result. Alerts for retest of infants who initially test HIV-negative are displayed on the top right hand corner. These alerts are triggered by the infant’s age corresponding to the Kenya guidelines for retesting at 9 and 18 months.
Procedures
EID care providers informed HIV-infected mothers during their first EID visit about the purpose of the HITSystem in a language they understood. Mothers provided verbal consent (99%) or opted to receive the current standard of care (paper-based recording without action alerts or communication). The primary EID provider entered mother–infant clinical data in the HITSystem at time of enrolment to trigger prospective action alerts. This process requires approximately 5–10 min per patient. The research protocol was approved by KEMRI and University of Kansas Medical Center ethics review boards.
We reviewed every EID patient file from the 12 months prior to deployment of the HITSystem at the peri-urban hospital. Due to a significantly higher volume at the urban site, we used a random starting point and selected every third file for analysis. Infant file numbers were arranged by date of arrival in the EID registries. This retrospective record review combined sources to maximize the completeness of preintervention data, including infants’ medical records, HEI Registry, KEMRI/NASCOP’s PCR testing database and HIV treatment records. All relevant data were reviewed by two study team members and clinical staff.
Retention was assessed as the proportion of infants engaged in EID care by 9 months postnatal, among those who were age and/or serostatus-eligible to receive interventions in the cascade of care per national EID guidelines. HIV status was established by HIV DNA PCR (Roche Amplicor version 1.5), which has 98% sensitivity and 99.7% specificity at or beyond 6 weeks postnatal age [15]. HIV antibody tests were used for infants more than 9 months of age and positive HIV antibody tests were confirmed with a HIV DNA PCR test.
Initiation of ART was determined by clinical notes and documentation of enrolment in the ART programme, designated by a CCC enrolment number. Turn-around time was assessed by comparing the number of weeks to complete the testing and notification cycle and the number of days between notification of a HIV-positive test result and ART initiation. Preintervention processes did not systematically notify mothers to return for follow-up appointments; therefore, the date of mother notification was estimated by using the date of the earliest patient visit after test results were received from the laboratory. If there was no record of a subsequent visit, we concluded that the mother was not informed of her infant’s results.
Statistical analyses
We calculated Q-Q plots and Shapiro–Wilk tests for each continuous variable to assess the distribution of data. Missing data were counted as loss to follow-up, as reflected in the denominators presented at each subsequent step of the EID cascade of care. Normally distributed variables are presented as mean/standard deviation (SD) and compared with t-tests. Continuous variables with nonparametric distributions are presented as median/interquartile range (IQR) and compared by Mann–Whitney U tests. Categorical variables were presented as proportions, using Pearson’s Chi-square tests or Fisher’s exact tests to compare pre and postintervention values. Attrition (not completing all 8 EID intervention points) is a major outcome of interest; thus, ‘missing’ or ‘lost’ dyads were included in outcome measures. All tests were two-sided, with alpha set to 0.05. Data were analysed using STATA Intercooled Version 10 (StataCorp LP, College Station, Texas, USA).
Results
We enrolled 386 HIV-infected mothers and their infants in the HITSystem pilot at the urban maternity hospital in Nairobi, and 137 mother–infant pairs at the peri-urban district hospital in western Kenya. Less than 1% declined participation. These two cohorts were compared with 222 and 98 historical control dyads from the two hospitals, respectively. Baseline characteristics of mother–infant pairs in the control and intervention groups at both hospitals were similar with respect to maternal age, infant sex, infant feeding method and infant HIV infections (Table 1). At the urban hospital, antenatal PMTCT utilization increased between the control and intervention periods. A majority of mothers in each setting used mobile phones.
Table 1.
Baseline characteristics.
| Urban Maternity Hospital, Nairobi
|
Peri-urban District Hospital, western Kenya
|
|||||
|---|---|---|---|---|---|---|
| Control April/10–March/11 |
HITSystem April/11–March/12 |
P | Control July/10–June/11 |
HITSystem July/11–June/12 |
P | |
| Maternal age, mean years (SD) | 29.9 (SD = 7.7) | 28.2 (SD = 5.3) | 30.1 (SD = 5.6) | 29.5 (SD = 5 9) | 0.46 | |
| Mother received PMTCTa | 192/222 (86.5) | 374/386 (96.8) | <0.001 | 59/98 (60.2) | 88/137 (64 2) | 0.58 |
| Infant sex (Male) | 108/216 (50.0) | 193/361 (53.5) | 0.45 | 46/96 (47 9) | 60/128 (46 9) | 0.87 |
| Infant-feeding methodb | ||||||
| Exclusive breast feeding | 133/152 (87.5) | 326/386 (84.4) | 0.59 | 65/76 (85 5) | 112/128 (87 5) | |
| Formula feeding | 16/152 (10.5) | 46/386 (11.9) | 5/76 (6 6) | 4/128 (3 1) | 0.51 | |
| Mixed feeding | 3/152 (1.9) | 14/386 (3.6) | 0.78 | 6/76 (7 9) | 12/128 (9.4) | |
| Positive HIV DNA PCR resultc | 6/151 (3.9) | 11/386 (2.9) | 13/86 (15 1) | 14/135 (10 4) | 0.22 | |
| Mobile phone utilizationd | n/a | 384/386 (99.5) | n/a | 92/137 (67 1) | ||
Data are n/N (%) unless otherwise stated.
PMTCT in this case refers to any antenatal treatment regimen.
Obtained at time of enrolment in EID.
Initial HIV DNA PCR result, average age at first test was 7.3 (control), 6.4 (intervention) weeks.
Mobile phone utilization was not assessed among historical controls.
Retention throughout the early infant diagnosis cascade of care
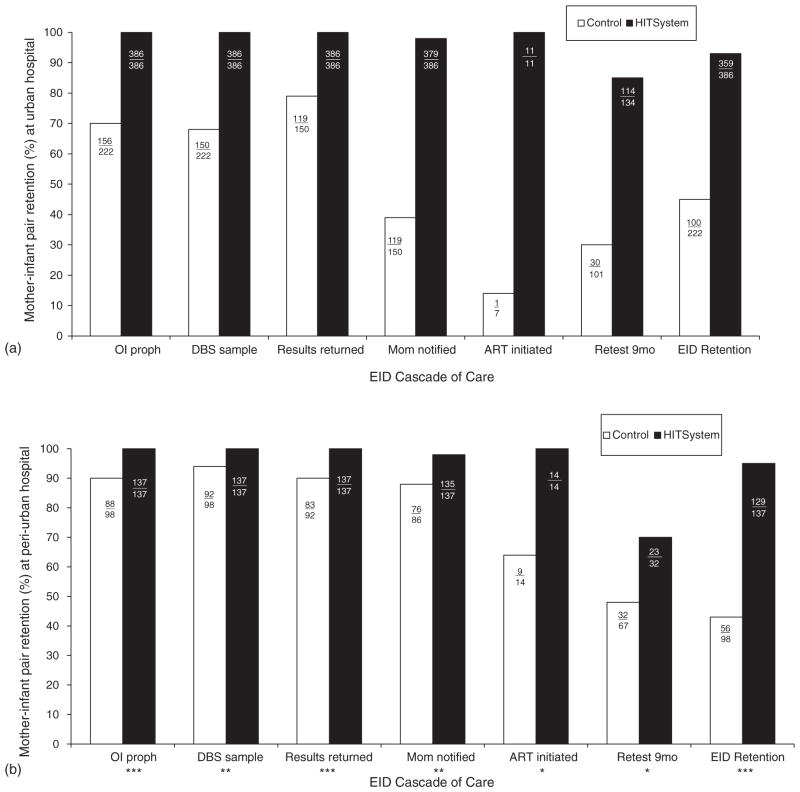
To determine whether the HITSystem improved longitudinal programme retention rates, we compared the proportion of mother–infant pairs still enrolled at each step of the EID cascade between control and intervention periods. Retention in EID care at 9 months more than doubled at both hospitals [45.1 vs. 93.0% (urban) and 43.2 vs. 94.1% (peri-urban), both P <0.001]. The HITSystem significantly improved retention at each point of the EID cascade at both hospitals, including increased provision of opportunistic infection prophylaxis (co-trimoxazole), collection of DBS samples, return of PCR results, notification of mothers regarding test results, initiation of ART among infants diagnosed HIV-infected and retesting of uninfected infants at 9 months of age (all P <0.05; Fig. 3). The HITSystem dramatically improved the proportion of HIV-infected infants initiated on ART [14 vs. 100% (urban) and 64 vs. 100% (peri-urban), both P <0.001].
Fig. 3. Retention of mother–infant pairs at each point in the early infant diagnosis cascade of care.
These data illustrate changes in the number of mother–infant pairs retained between historical controls and during the HITSystem intervention at the urban (3A) and peri-urban (3B) hospitals. The y-axis represents percentage of mother–infant pairs retained at each sequential point along the EID cascade of care (x-axis). Raw numbers are listed within the bar graph for each outcome, and the denominators reflect numbers eligible for each subsequent step in the cascade. All comparisons in 3A are significant at P <0.001. The following symbols illustrate the level of statistical significance in 3B:* P <0.05, **P <0.01, ***P <0.001.
Meeting time-sensitive targets and improving turn-around time for results
Quality EID requires not only retention of patients in care but also provision of interventions within time-sensitive targets. The HITSystem significantly improved all time-sensitive quality measures in one or both settings (Table 2). Compared with the control population, infants enrolled in the HITSystem were more likely to receive opportunistic infection prophylaxis by 6 weeks postnatal at both hospitals (P <0.001). Complete turn-around time for the communication cycle of test results (DBS collection to mother notification) was significantly lower among those enrolled in the HITSystem [median 5.0 vs. 6.3 weeks (urban) and 3.4 vs. 8.1 weeks (peri-urban), both P <0.001].
Table 2.
Time-sensitive targets and turn-around times for early infant diagnosis.
| Urban Maternity Hospital
|
Peri-urban District Hospital
|
|||||
|---|---|---|---|---|---|---|
| Control April/10–March/11 N = 222 |
HITSystem June/11–June/12 N = 386 |
P | Control June/10–May/11 N = 98 |
HITSystem June/11–June/12 N = 137 |
P | |
| Meeting time-sensitive targets | ||||||
| OI prophylaxis by 6 weeks | 34/61 (55.7) | 337/386 (87.3) | <0.001 | 29/88 (32.9) | 108/137 (78.8) | <0.001 |
| Median age OI started (weeks) | 6.6 (6.0–7·9) | 6·0 (6.0–6·0) | <0.001 | 9.9 (6.1–13.6) | 6.0 (6.0–6.0) | <0.001 |
| First DBS by 6 weeks | 99/150 (66.0) | 202/275 (73 4) | 0.07 | 34/96 (35.4) | 73/137 (53.3) | 0.007 |
| Median age at 1st DBS | 6.5 (6.1–7.7) | 6.3 (6.0–7.3) | 0.03 | 8.1 (6.1–13.8) | 6.6 (6.1–11.3) | 0.38 |
| Turn-around times | ||||||
| DBS sample to PCR results (weeks) | 3.4 (2.1–5.0) | 3.5 (2.1–4.8) | 0.32 | 3.6 (2.7–5·0) | 2.1 (1.3–2.8) | <0.001 |
| Results to notification of mom (weeks)a | 4.0 (2.3–10.0) | 1.3 (1.0–1.6) | <0.001 | 3.7 (1.7–8.0) | 1.6 (1.1–2.1) | <0.001 |
| Complete turn-around-time (weeks)b | 6.3 (4.7–13·6) | 5.0 (3.7–6.1) | <0.001 | 8.1 (4.9–12.9) | 3.4 (2.8–4.7) | <0.001 |
| Notification to ART initiation (days)c | 40 | 13 (12–29) | 36 (33–77) | 1 (0–2.1) | <0.001 | |
Data presented as n/N (%), and continuous data as median (IQR), IQR = Q1–Q3.
Documentation of mother notification was very limited among historical controls, thus the denominators for notification, and consequently ‘complete turn-around time’, were small [n = 44 (urban control), and n = 70 (peri-urban control)].
Includes time from sample collection to notification of mother with PCR test results.
Only one HIV-infected infant in the control group at the urban hospital was started on ART.
Increasing early antiretroviral therapy initiation among HIV-infected infants
At the urban hospital, only one of seven HIV-infected infants in the control period was initiated on ART, compared with 11 of 11 during the HITSystem intervention (Fig. 3). ART initiation occurred 40 days after mother notification during the control period, compared with 13 median days during the HITSystem intervention. In the peri-urban setting, median days for initiating ART decreased from 36 days to 1 day (P <0.001). These data confirm that the HITSystem is associated with significant improvements in retention, efficiency and early initiation of ART in the context of EID care.
Discussion
Implementation of the HITSystem was associated with more than a two-fold increase in both EID retention and initiation of ART among HIV-infected infants compared to historical controls. Furthermore, turn-around time for PCR test results significantly improved, and the time for ART initiation among HIV-infected infants was reduced from 38 to 7 median days. Notably, the HITSystem improved care in both the urban and peri-urban settings, despite different enrolment volumes and known regional variation in perinatal HIV transmission rates. Similarly, the HITSystem improved communication of results and ART initiation in the urban setting despite the challenges of a large catchment area and transient patient population. In short, the HITSystem’s novel integration of customized alerts (Internet and SMS-based) with dedicated prospective tracking proved to be feasible and effective in both settings (urban, peri-urban) and in both types of hospitals (large maternity and district).
The HITSystem intervention successfully addresses multiple implementation barriers for the EID cascade of care identified in a recent systematic review by Ciaranello et al. [6]. Specifically, historical global data indicate that only 64–78% of PCR test results are returned to health facilities [16,17] (turn-around time of 1–3 months) [18], between 37 and 81% of mothers are notified of these results [12,16], and only 26–59% of HIV-infected infants ever initiate ART [6,16,18,19]. The HITSystem achieved outcomes far superior to those previously observed in the literature, with 100% of PCR results returned to health facilities, 98% of mothers notified of results (average turn-around time of 1.4 weeks) and 100% of HIV-infected infants initiated ART. Importantly, the HITSystem also improved clinical management by initiating 100% of HIV-exposed infants on opportunistic infection prophylaxis, compared with only 35–66% in other studies [6,20].
The HITSystem capitalizes on the now near-ubiquitous access to mobile phones, increased availability of the Internet and emergence of health interventions using mobile technology in several contexts, including HIV and malaria control to improve clinical (e.g. provider practices, medication adherence) [21–24] and health systems outcomes (e.g. appointment reminders and improved turn-around time for results) [25]. In 2012, van Velthoven et al. [21] contributed a systemic review of the scope and effectiveness of mobile phone messaging for HIV/AIDS care. Among the 21 HIV-related studies that met inclusion criteria, none addressed EID. The HITSystem addresses this gap by connecting multiple EID stakeholders in an interactive real-time feedback system that sends alerts for action until resolved. Accountability for key steps in the EID cascade is thus transferred into the hands of mothers, providers and lab technicians while the system monitors progress to identify and resolve persistent challenges. These innovations allow expedited test results that inform infant-feeding decisions [26] and postnatal ART recommendations [27] to maximize quality of infant care, while improved retention at 9 months permits detection of interval transmissions that have occurred due to breastfeeding, thereby avoiding missed opportunities to identify and treat.
To our knowledge, the HITSystem is the first to merge Internet and SMS technologies to connect multiple EID stakeholders and evidence dramatic improvements in the quality of clinical services by addressing multiple implementation barriers. As such, it represents a technological advance beyond previous attempts that focused on the singular goal of improving turn-around times for PCR results (using either text messaging [28] or one-way SMS printers [29]) or increasing enrolment [30].
Feasibility and sustainability
HITSystem implementation is feasible in remote areas, as it requires neither a continuous supply of electricity nor wire-based Internet access. The only requirement is that the site is able to pick up mobile broadband, which is accessible in nearly all Kenyan hospitals. To evaluate a sustainable implementation strategy, only existing clinical and laboratory staff were trained to use the HITSystem.
Careful training and early one-on-one support ensured that existing EID and laboratory staff were able to integrate the HITSystem into their routine clinical workflow, reducing the perception of increased burden. Over time, some staff reported that the HITSystem data facilitated the required reporting for the Ministry of Health (MOH). Although managed by existing hospital and laboratory staff, HITSystem utilization and quality assurance can be monitored by off-site personnel, thus reducing costs of technical assistance and travel.
Although implementation costs vary by hospital according to the existing personnel and infrastructure, the only fixed monthly costs include a $200 SMS and secure data storage fee, and approximately $50 for mobile broadband minutes for Internet connection and use. One-time startup costs include initial and follow-up training, a modem and a computer, if needed. These costs make widespread implementation of the HITSystem both feasible and sustainable, even in low-resource settings. Furthermore, the HITSystem reduces wasted resources by maximizing the return on the MOH’s significant investment in providing PCR testing by ensuring that mothers receive test results and that indicated treatments are rapidly initiated. As Kenya moves towards a computer-based health information system supported by the MOH [31], care has been taken to ensure that the HITSystem’s software can be integrated to complement national systems, and be adapted for other populations and health outcomes.
Limitations
The current version of the HITSystem is limited by its enrolment process that relies on mothers to bring their infants for EID, thus missing HIV-exposed infants who never present for care. Current efforts to expand the scope of the HITSystem to enrol HIV-infected pregnant women at their first PMTCT visit and follow them postpartum will permit opportunities to enrol HIV-exposed infants at birth and promote even earlier interventions. Limitations of the observational study design include the inability to demonstrate causality, the potential for covariate imbalance between populations in the historical control group, unknown or unmeasured covariates that may have influenced the study outcomes (i.e. improvement in service delivery over time) and restricted generalizability of pilot findings to urban and peri-urban government hospitals in Kenya (although comparable results might be expected in similar settings in other countries). Similarly, limited and inconsistent documentation of EID outcomes in the control period resulted in significantly smaller denominators for time-related variables in the control populations. To our knowledge, there were no other EID-focused interventions (i.e. trainings or programmes) at the two HITSystem pilot sites during the study period that would have influenced EID outcomes.
Future steps
On the basis of these pilot findings, Global Health Innovations and KEMRI are working with NASCOP to strategize scale-up of the HITSystem in Kenya. These data reveal areas for improvement, including strategies to enrol infants earlier and further reduce turn-around times by improving patient follow-up and tracing strategies. We have begun to evaluate the impact of the HITSystem at 18 months follow-up, cost-effectiveness analyses distinguishing research and programme costs, and qualitative evaluations with mothers to better understand how to increase confidence in the system and minimize barriers. Similar interviews with EID and lab providers continue to highlight system improvements prior to widespread dissemination. If implemented with diligence, the HITSystem has the potential to revolutionize EID and clinical management of HIV-exposed infants globally.
Acknowledgments
We are grateful to the mothers and infants who participated in the HITSystem intervention and the clinical staff who were integral to these efforts. We acknowledge members of the Kenya HITSystem Team and partners: Julie Dougherty, Irene Odera, Rael Odeke, Dr Jonah Maswai, Dr Teresa Simiyu, Anthony Naibei, Sharon Koech, Dr Lazarus Omondi, Alice Onyino, Patrick Mwinamo, Dr Edward Serem, Pamela Wawire, Mary Mutai, Emmie Kavai and Charles Bawcom. We also acknowledge the critical role of our government partners at NASCOP; Nancy Bowen, Dr Martin Sirengo, Dr Irene Mukui, Dr Ibrahim Mohamed and Dr William Maina. We are also appreciative of the support of KEMRI Director, Prof. Solomon Mpoke and his Deputy Director of Research, Dr Elizabeth Bukusi, and of research mentors Drs. Andy Ruff and Michael Sweat. Without the generous pro-bono contributions of HITSystem software developers at OnTarget LLC, Terry Oehrke and Brian Hickey, these efforts would not have been possible. We thank Dr Niaman Nazir and George Pro for their role in data management, and are also grateful to Mr. Jay O’Brien who graphically designed Fig. 1. These efforts were funded by Global Health Innovations and Health Empowering Humanity through private donations, and the National Institutes of Child Health and Development, R01HD076673.
Funding for this study was provided by Global Health Innovations and Health Empowering Humanity through private donations, and the National Institutes of Child Health and Development, R01HD076673.
Footnotes
Conflicts of interest
We declare no conflicts of interest.
B.J.G. designed the concept of the HITSystem intervention with software programmers from On Target. S.F.K., B.J.G., S.K. and V.O. designed the HITSystem pilot study protocols and assisted with training and implementation with the HITSystem Team. V.O., H.D.O. and members of the HITSystem Team assisted with data collection, and S.F.K. conducted statistical analyses with technical guidance from G.A.P. G.A.P., K.G., J.K.S., S.K., V.O., A.M. and D.K. provided strategic framing of the study findings in the context of global and Kenya-specific EID efforts. All authors contributed to the writing and refinement of the manuscript.
References
- 1.UNICEF. [Accessed May 7, 2012];State of the worlds children. 2010 http://www.unicef.org/rightsite/sowc/
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendergast A, Mphatswe W, Tudor-Williams G, Rakgotho M, Pillay V, Thobakgale C, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Antiretroviral therapy for infants and children: report of the WHO Technical Reference Group. Paediatric HIV/ART Care Guidelines Group Meeting; 2010; Geneva: World Health Organization; [Google Scholar]
- 6.Ciaranello A, Park J, Ramirez-Avila L, Freedberg K, Walenshy R, Leryo V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. [Accessed June 22, 2013];Antiretroviral therapy for HIV infection in infants and children: recommendations for a public health approach. 2010 http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed]
- 8.Kenya Ministry of Health. Algorithm for early diagnosis of HIV in children. Ministry of Public Health and Sanitation; Nairobi, Kenya: Dec, 2009. [Google Scholar]
- 9.Ministry of Health. [Accessed December 4, 2013];National AIDS/STD Control Programme. (NASCOP) Kenya EID summary. 2012 http://www.nascop.org/eid/overall.php.
- 10.World Health Organizations, UNAIDS, UNICEF. [Accessed February 9, 2014];Global HIV/AIDS response; epidemic update and health sector progress report toward universal access. 2011 http://www.unicefusa.org/assets/pdf/2011HIVreport_opt.pdf.
- 11.Cherutich P, Inwani I, Nduati R, Mbori-Ngacha Optimizing paediatric HIV care in Kenya: challenges in early infant diagnosis. [Accessed April 11, 2013];WHO Bulletin. doi: 10.2471/BLT.07.040402. http://www.who.int/bulletin/volumes/86/2/07-040402/en/index.html. [DOI] [PMC free article] [PubMed]
- 12.Creek T, Sherman G, Nkengagong J, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197 (Suppl 3):S64–S71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Hassan AS. Dynamics and constraints of early infant diagnosis of HIV Infection in rural Kenya. AIDS Behav. 2012;16:5–12. doi: 10.1007/s10461-010-9877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Epidemic update and health sector progress towards universal access. Geneva: WHO, UNICEF, UNAIDS; 2011. Progress report 2011: global HIV/AIDS response. [Google Scholar]
- 15.Mphatswe W, Blanckenberg N, Tudor-Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21:1253–1261. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 16.Kimario CJ, Schimana W, Charles D, Teri IE, Giphart A, Marlinc R, et al. Scale up of early infant diagnosis (EID) in Tanzania: experience from the Elizabeth Glaser Pediatric AIDS Foundation [Abstract 887; The 2009 HIV/AIDS Implementers’ Meeting; June 10–14, 2009; Windhoek, Namibia. [Accessed June 5, 2014]. p. 79. http://www.webcitation.org/5zwBc2qmW. [Google Scholar]
- 17.Sundaram M, Lukhele B. Identification patient loss points from testing to treatment initiation among infants tested in Swaziland [Abstract no. MOPDD103]. 5th IAS Conference on HIV Pathogenesis and Treatment; July 19–22, 2009; Cape Town, South Africa. [Accessed June 5, 2014]. http://www.iasociety.org/Default.aspx?search=MOPDD103&pageId=7. [Google Scholar]
- 18.Khamadi S, Okoth V, Lihana R, Nabwera J, Hungu J, Okoth F, et al. Rapid identification of infants for antiretroviral therapy in a resource poor setting: the Kenya experience. J Trop Pediatr. 2008;54:370–374. doi: 10.1093/tropej/fmn036. [DOI] [PubMed] [Google Scholar]
- 19.Mahdi MA, Chouraya C, Waligo A, Shabalat F, Kieffer MP. Early infant diagnosis (EID): Swaziland experience [Abstract no. MOPE0223; AIDS 2008 – XVII International AIDS Conference; August 3–7, 2008; [Accessed June 5, 2014]. p. 104. http://www.iasociety.org/Default.aspx?search=MOPE0223&pageId=7. [Google Scholar]
- 20.Moodley D, Reddy L, Mahungo W, Masha R. Factors associated with coverage of cotrimoxazole prophylaxis in HIV-exposed children in South Africa. PLoS One. 2013;8:e63273. doi: 10.1371/journal.pone.0063273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Velthoven MH, Brusamento S, Majeed A, Car J. Scope and effectiveness of mobile phone messaging for HIV/AIDS care: a systematic review. Psychol Health Med. 2013;18:182–202. doi: 10.1080/13548506.2012.701310. [DOI] [PubMed] [Google Scholar]
- 22.Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet. 2011;378:795–803. doi: 10.1016/S0140-6736(11)60783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretro-viral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 24.Déglise C, Suggs LS, Odermatt P. SMS for disease control in developing countries: a systematic review of mobile health applications. J Telemed Telecare. 2012;18:273–281. doi: 10.1258/jtt.2012.110810. [DOI] [PubMed] [Google Scholar]
- 25.Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, et al. The effectiveness of mobile-health technologies to improve health-care service delivery processes: a systematic review and meta-analysis. Cochrane Database Syst Rev. 2012;7:CD007458. doi: 10.1371/journal.pmed.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. [Accessed March 12, 2013];Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. http://www.who.int/child_adolescent_health/documents/9789241599535/en/index.html. [PubMed]
- 27.Persaud D, Bedri A, Ziemniak C, Moorthy A, Gudetta B, Abashawl A, et al. Ethiopian Swen Study Team. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retroviruses. 2011;27:823–829. doi: 10.1089/aid.2010.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidenberg P, Nicholson S, Schaefer M, Semrau KJ, Bweupe M, Masese N, et al. Early infant diagnosis of HIV infection in Zambia through mobile phone texting of blood test results. Bull World Health Organ. 2012;90:348–356. doi: 10.2471/BLT.11.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabo U, Onotu D, Miral M. SMS printer pilot programme for Early Infant Diagnosis (EID) of HIV-exposed infants for PMTCT in Kano, Nigeria. 6th International Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20, 2011; Rome, Italy. [Accessed June 5, 2014]. http://pag.ias2011.org/Abstracts.aspx?AID=713. [Google Scholar]
- 30.Ciampa PJ, Tique JA, Jumá N, Sidat M, Moon TD, Rothman RL, Vermund SH. Addressing poor retention of infants exposed to HIV: a quality improvement study in rural Mozambique. J Acquir Immune Defic Syndr. 2012;60:e46–e52. doi: 10.1097/QAI.0b013e31824c0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health, Kenya. [Accessed November 20, 2013];Health sector strategic plan for health information system, 2009–2014. http://apps.who.int/healthmetrics/library/countries/HMN_KEN_StrPlan_Final_2010_02_en.pdf.