FIGURE 2.
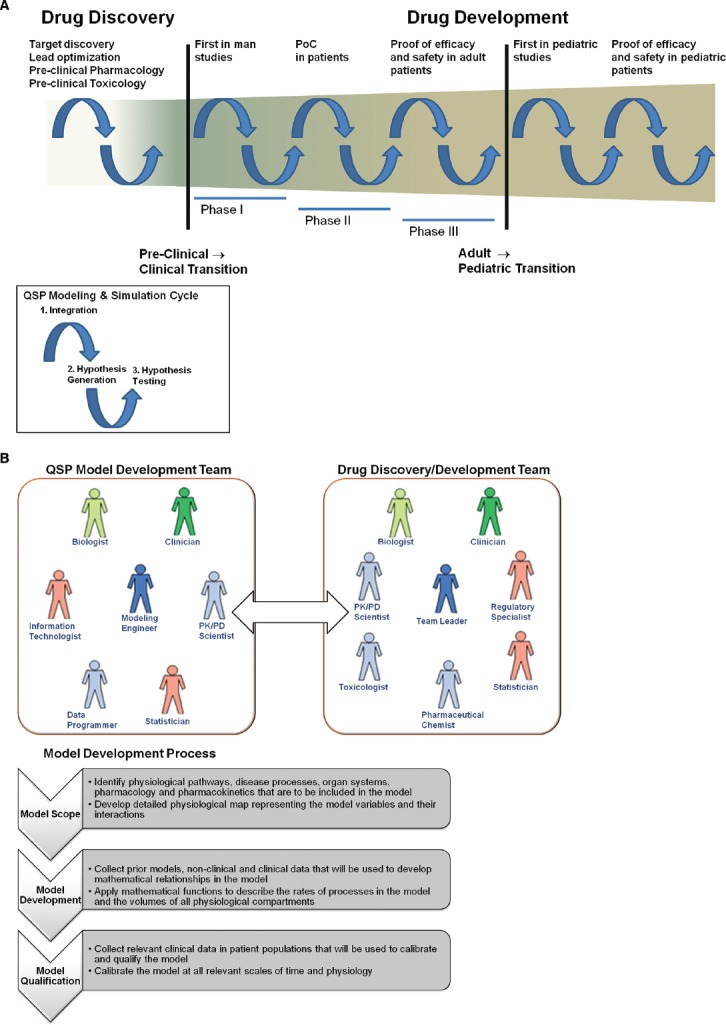
(A) Incorporation of Quantitative Systems Pharmacology modeling and simulation in pharmaceutical R&D. Drug discovery and development is a long and complex process with numerous transition periods where effective translation from one experimental model to the next is a challenge. Major transitions occur when moving to first in man studies and first in pediatric studies. The cycles of application of QSP modeling and simulation are defined by (1) integration of experimental data and biological knowledge to develop QSP models; (2) generation of hypotheses for potential outcomes in future experiments; (3) testing of those hypotheses with experiments that have been designed via simulation from QSP models. (B) Integration of QSP models in pharmaceutical R&D process. The model development team should be a sub-team of existing drug discovery and development teams. The goals of the model development team are to develop QSP models for their particular disease area and/or to apply existing QSP models to facilitate key milestones in discovery and development. The model development process should be rigorous and stepwise, so that models that are developed can used broadly in the disease area and can be used to communicate with regulatory agencies.
