Figure 9.
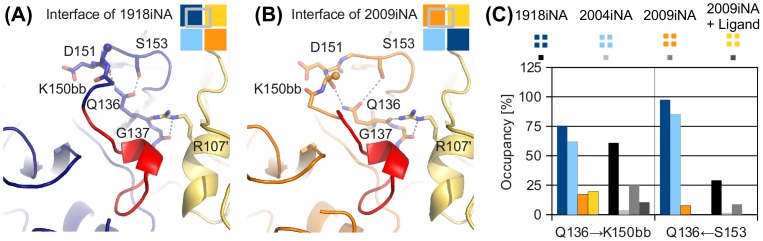
Inter-subunit interactions propagate toward the iNA active site via glutamine Q136. This conserved amino acid interacts with R107’ of the neighboring subunit (yellow) and the 150-loop residues including active site aspartic acid D151 via hydrogen bonds with the backbone oxygen of K150 and the side chain of S153 (gray lines). Different starting conformations of the 150-loop in 1918iNA (A, open 150-loop) and 2009iNA (B, closed 150-loop) are reflected in altered hydrogen bond occupancy rates for the Q136 side chain. (C) Occupancy of Q136 side chain interactions in tetramer and monomer simulations show that the tetrameric assembly state stabilizes hydrogen bonds between Q136 and two 150-loop residues, K150, and S153. Arrows are pointing from donor to acceptor residues.
