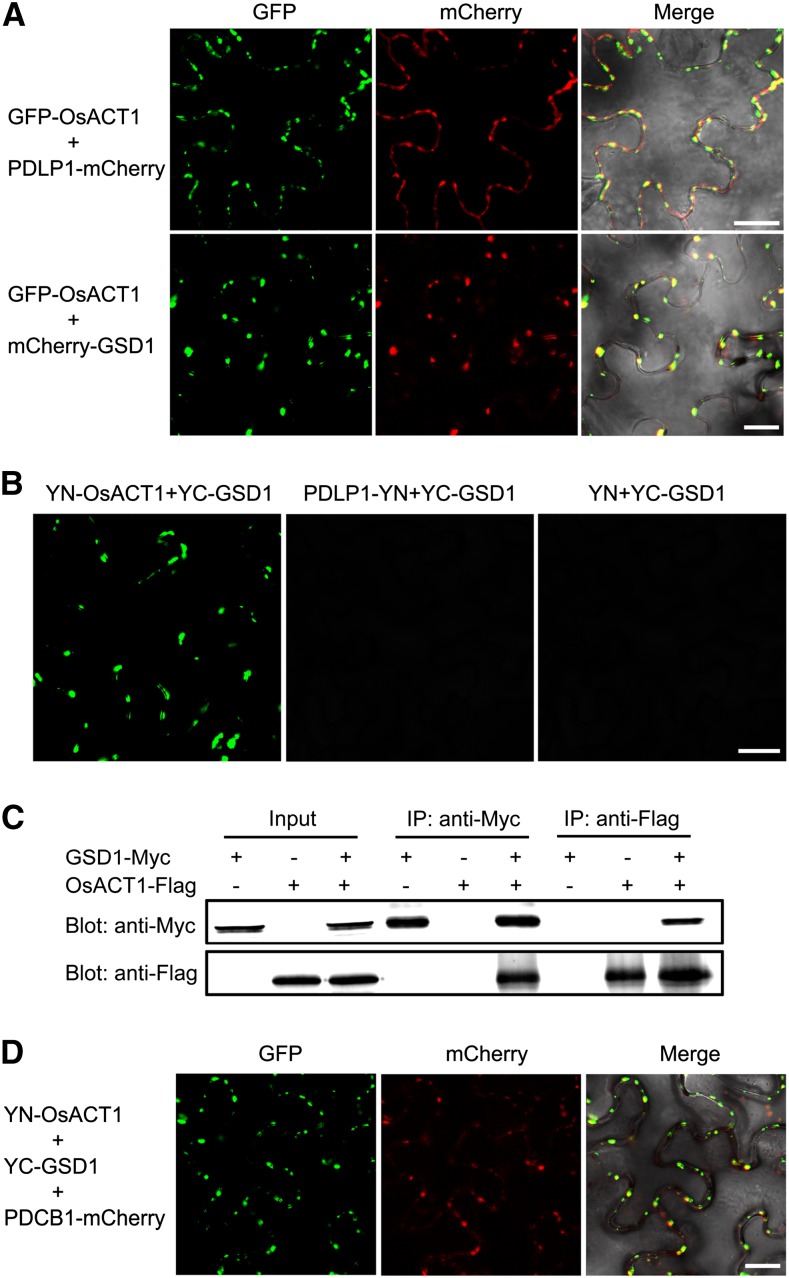
Figure 9.
GSD1 interacts with OsACT1 at PD. A, mCherry-GSD1 and PDLP1-mCherry were cotransformed with GFP-OsACT1 into tobacco leaves, respectively. Confocal images show overlapping OsACT1 GFP fluorescence signals and PD marker protein PDLP1 mCherry signals. OsACT1 GFP signals and GSD1 mCherry signals also overlap. B, Interaction of GSD1 with OsACT1 was detected by BiFC. YN-OsACT1 (YN, N-terminal part of YFP), PDLP1-YN, and YN were cotransformed with YC-GSD1 (YC, C-terminal part of YFP) into tobacco leaves, respectively. YFP signals were detected in YN-OsACT1 and YC-GSD1 coexpressed tobacco leaves. No YFP fluorescence signal was detected in PDLP1-YN and YC-GSD1 or YN and YC-GSD1 coexpressed tobacco leaves. C, Interaction between GSD1-Myc and OsACT1-Flag was detected by Co-IP. GSD1-Myc and OsACT1-FLAG were expressed or coexpressed in tobacco leaves. Total protein extracts were separately immunoprecipitated with anti-Myc antibody-coupled agarose beads and anti-Flag antibody-coupled agarose beads. Proteins from the crude lysates and immunoprecipitated proteins were detected with anti-Myc antibodies and anti-Flag antibodies, respectively. D, YN-OsACT1, YC-GSD1, and PDCB1-mCherry were cotransformed into tobacco leaves. YFP and mCherry signals were detected using a confocal microscope. Overlaid YFP and mCherry fluorescence signals indicate that the GSD1/OsACT1 complex colocalized with PD callose binding protein PDCB1.