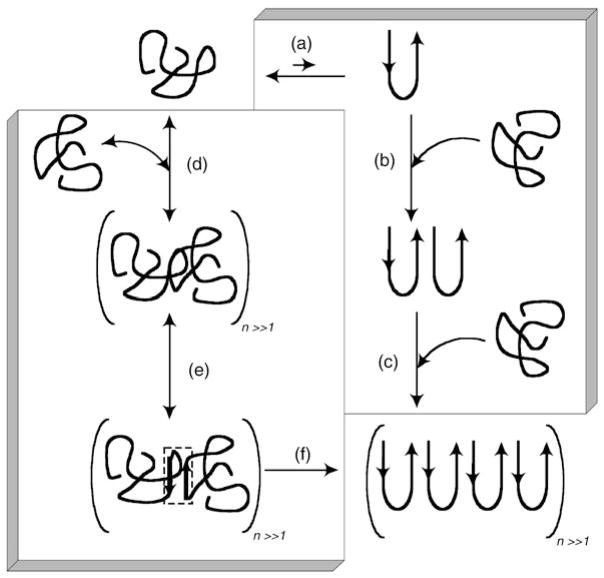
Figure 10.2.
Proposed mechanism for polyQ peptide aggregation from an unfolded monomer to an insoluble fibrillar aggregate. The right-hand side shows the nucleation–elongation mechanism (steps (a) to (c)). a A monomer is in rapid equilibrium with a thermodynamically unfavorable β-sheet nucleus, b The β-Sheet nucleus serves as a template for the addition of a monomer. c Fibrils elongate in repeated rounds of monomer addition. The left-hand side outlines the association–conformational conversion mechanism (steps (d) to (f)). d Monomers lacking regular secondary structure rapidly associate into large soluble oligomers, driven by hydrophobic interactions, e Conformational rearrangement within the large oligomers leads to the formation of β-sheet nodes (indicated by the small dashed box), f β-Sheet formation propagates throughout the oligomers, producing insoluble fibrillar aggregates