Abstract
Phosphorylation is considered a key event in the signalling and regulation of the μ opioid receptor (MOPr). Here we used mass spectroscopy to determine the phosphorylation status of the C-terminal tail of the rat MOPr expressed in HEK-293 cells. Under basal conditions, MOPr is phosphorylated on Ser363 and Thr370, while in the presence of morphine or [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO), the COOH-terminus is phosphorylated at three additional residues, Ser356, Thr357, and Ser375. Using N-terminal Glutathione S Transferase (GST) fusion proteins of the cytoplasmic, C-terminal tail of MOPr and point mutations of the same, we show that, in vitro, purified G protein-coupled receptor kinase 2 (GRK2) phosphorylates Ser375, PKC phosphorylates Ser363 whilst CaMKII phosphorylates Thr370. Phosphorylation of the GST fusion protein of the C-terminal tail of MOPr enhanced its ability to bind arrestin-2 and -3. Hence, our study identifies both the basal and agonist-stimulated phospho-acceptor sites in the C-terminal tail of MOPr, and suggests that the receptor is subject to phosphorylation and hence regulation by multiple protein kinases.
Keywords: μ opioid receptor, mass spectrometry, phosphorylation, kinases, arrestins, desensitization
Introduction
Phosphorylation of MOPr has been implicated in the desensitization and trafficking of the receptor, as well as in the development of opioid tolerance in the intact organism (Johnson et al. 2005; Dang and Christie 2012; Kelly et al. 2008; Kelly 2011). The identity of phosphorylated amino acids in the intracellular regions of MOPr is therefore of considerable interest and importance. Using a [32P]orthophosphate metabolic prelabelling approach, in conjunction with site-directed mutagenesis, previous studies have identified amino acids in MOPr that are phosphorylated under basal conditions or in response to opioid agonist (Deng et al. 2000; El Kouhen et al. 2001; Wang et al. 2002; Schulz et al. 2004; Ozsoy et al. 2005; Wang et al. 2007; Chu et al. 2008). However there remains disagreement as to the identity of phosphorylated residues, as well as the role of such residues in the regulation of MOPr (Pak et al. 1997; Burd et al. 1998; Deng et al. 2000; El Kouhen et al. 2001; Wang et al. 2002; Schulz et al. 2004; Johnson et al. 2005; Ozsoy et al. 2005; Wang et al. 2007; Chu et al. 2008). This may in part be due to the previous reliance on the use of MOPr point mutations to confirm phosphorylation data. Such mutations might influence receptor function in ways that are not related to the phosphorylation of that particular residue (Kim et al. 2005). A better approach would be the direct identification of phosphorylated residues in the receptor by mass spectrometry. This has been achieved for some G protein-coupled receptors (GPCRs) including the β2-adrenoceptor (Trester-Zedlitz et al. 2005; Nobles et al. 2011), the V2 vasopressin receptor (Wu et al. 2008) and the CXCR4 receptor (Busillo et al. 2010). In the case of opioid receptors, mass spectrometry has been employed to sequence the purified MOPr and KOPr (Christoffers et al. 2003; Wannemacher et al. 2008). Very recently it has also been applied to study the phosphorylation sites of the C-terminal tail of MOPr (Lau et al. 2011; Feng et al. 2011; Moulédous et al. 2012).
Apart from the identification of phospho-acceptor sites, the kinases that mediate these phosporylatyion events are generally not known. However G protein-coupled receptor kinases have been implicated in the phosphorylation of Ser375 (Schulz et al. 2004). Here we use Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) to identify phosphorylated amino acids in the cytoplasmic, C-terminal tail of MOPr. We find that this region of MOPr is phosphorylated on multiple residues both under basal conditions and upon stimulation by agonist. Furthermore, using GST fusion proteins of the C-terminal tail of the receptor we identify, in vitro, possible sites for phosphorylation by both GRK2 and second messenger-dependent protein kinases. Finally, we demonstrate that phosphorylation of the C-terminal tail of MOPr increases its ability to interact with arrestins.
Materials and Methods
Cell culture
HEK293 cells stably expressing MOPr (~0.5 pmol/mg membrane protein) tagged at the NH2 terminus with hemagglutinin (HA) were grown in Dulbecco's Modified Eagle's medium (Invitrogen, Paisley, UK) supplemented with 10% foetal calf serum, 200 μg/ml geneticin (PAA, Pasching, Austria), 10 U/ml penicillin and 10 μg/ml streptomycin. For experimental purposes, cells were grown to approximately 90% confluence and incubated with 10 μM DAMGO, 30 μM morphine or with no drug for 10 min, washed twice in ice-cold phosphate buffered saline and scraped into lysis buffer (20 mM Tris/HCl pH7.4, 140 mM NaCl, 1 mM EDTA containing protease inhibitors (Complete; Roche Products, Welwyn Garden City, UK) and phosphatase inhibitors (PhosStop; Roche Products)). Unless otherwise stated, all drugs, chemicals and kits were obtained from Sigma-Aldrich (Poole, UK).
Affinity purification of HA-tagged MOPr and tryptic digestion
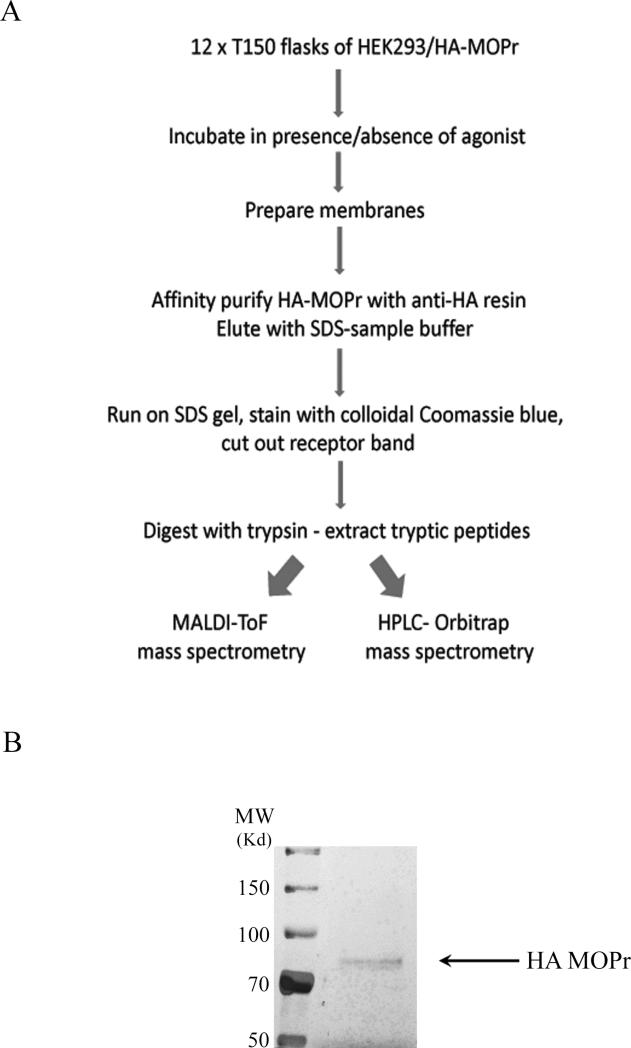
A flowchart outlining the methodology is shown in Fig. 1A. Cells from 12 × 150 cm2 flasks were scraped into 25 ml lysis buffer and lysed with a Polytron homogeniser. Nuclei and cell debris were removed by centrifugation at 3,000 g for 5 min, and the supernatant was diluted to 50 ml with lysis buffer and subjected to centrifugation at 40,000 g for 1 h. The resultant membrane pellet was washed with lysis buffer and then dissolved in 5 ml lysis buffer containing 1% of the detergent NP40. Insoluble material was removed by centrifugation and the supernatant diluted 2-fold with 20 mM Tris/HCl pH 7.4, 140 mM NaCl. MOPr was immunoprecipitated by mixing with agarose beads coupled to anti-HA antibody (200 μl) (Roche Products) at 4°C overnight. Beads were then washed three times with 20 mM Tris/HCl pH7.4, 140 mM NaCl, 0.5% NP40. Bound proteins were eluted into SDS sample buffer and subjected to SDS-PAGE. The gel was stained with colloidal Coomassie blue and the band corresponding to MOPr (~80kD) was excised. (For the purposes of photography one gel was stained using the ProteoSilver™ Plus Silver Stain kit (Bio-Rad laboratories, Hemel Hempstead, UK) according to the supplier's instructions; see Fig. 1B). The gel slice was washed three times for 15 min with 100 mM ammonium bicarbonate. Reduction and alkylation of cysteine residues was performed by incubation of the gel slice in 50 mM ammonium bicarbonate containing 10 mM dithiothreitol at 60°C for 30 min followed by incubation in 50 mM ammonium bicarbonate containing 100 mM iodoacetamide for 30 min in the dark. Gel slices were washed 3 times for 5 min with 50 mM ammonium bicarbonate containing 50% acetonitrile and incubated overnight at 37°C in 50 mM ammonium bicarbonate containing 1 μg sequencing grade trypsin (Promega, Southampton, UK). The solvent was removed in a under vacuum in a centrifugal concentrator and the peptides redissolved in 0.1% triflouroacetic acid.
Fig. 1.
(A) Flow diagram of methodology for MOPr purification and preparation for LC-MS/MS. (B) Gel stained with ProteoSilver™ Plus Silver Stain to demonstrate purity of the MOPr preparation.
LC-MS/MS
LC-MS/MS was carried out upon each sample using an LTQ (Linear Trap Quadrupole) Orbitrap mass spectrometer by the Protein and Nucleic Acid Chemistry Laboratory at the University of Leicester. Briefly, peptides resulting from in-gel digestion were loaded at high flow rate onto a reverse-phase trapping column (0.3 mm i.d. × 1 mm), containing 5 μm C18 300 Å Acclaim PepMap media (Dionex, Camberley, UK) and eluted through a reverse-phase capillary column (75 μm i.d. × 150 mm) containing Symmetry C18 100 Å media (Waters, Elstree, UK). The output from the column was sprayed directly into the nanospray ion source of the LTQ Orbitrap mass spectrometer. The resulting spectra were analysed against the UniProtKB/SwissProt database using MASCOT software (Matrix Science Ltd., London, UK) with peptide tolerance set to 5 ppm and the MS/MS tolerance set to 0.6 Daltons. Fixed modifications were set as carbamidomethyl cysteine with variable modifications of phosphoserine, phosphothreonine, phosphotyrosine and oxidised methionine. The enzyme was set to Trypsin/P and up to 2 missed cleavages were allowed. Peptides with a MASCOT score greater than 20 and where the probability (P) that the observed match was a random event was <0.05 were included in the analysis. The spectra of peptides reported as being phosphorylated were interrogated manually to confirm the precise sites of phosphorylation. For each condition (i.e. plus DAMGO or morphine) the experiment was conducted 3 times and the results presented as a summary in Fig. 2.
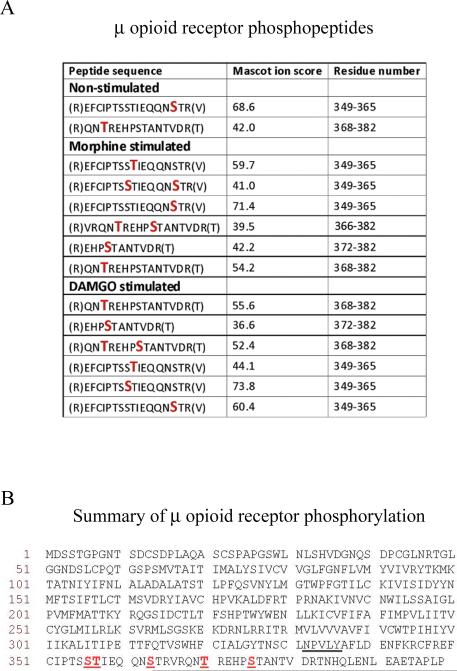
Fig. 2.
Identity of phosphorylated residues in MOPr. (A) MOPr phosphopeptides obtained in the presence or absence of morphine or DAMGO. Phosphorylated Ser or Thr residues are denoted by larger red letters. Spectra were analysed against the UniProtKB/SwissProt database using MASCOT software with peptide tolerance set to 5 ppm and the MS/MS tolerance set to 0.6 Daltons. The spectra of peptides reported as being phosphorylated were interrogated manually to confirm the precise sites of phosphorylation. (B) Summary of phosphorylation sites in the MOPr C-terminal tail. The phosphorylated Ser or Thr residues (Ser356, Thr357, Ser363, Thr370, and Ser375) are underlined, as is the NPXXY motif, at the base of the 7th transmembrane domain where the C-terminus begins. The mass spectrometry experiments described here were carried out three times for each condition. The data presented is a summary of these experiments and the spectra shown in Supplementary Fig. 1 are representative of the results obtained.
GST-fusion protein expression and purification
The Glutathione-S-transferase (GST) fusion proteins, including GST-rat MOPr-2nd intracellular loop (GST-2IL), GST-rat MOPr-3rd intracellular loop (GST-3IL), and GST-rat MOPr C-terminal tail (GST-CT) were expressed in the pGEX-4T-1 vector. The sequences for MOPr-2IL, -3IL, and -CT were, respectively, S162VDRYIAVCHPVKALDFRTPRNAK185; L257RLKSVRMLSGSKEKDRNLRRITRMV282; N328SCLNPVLYAFLDENFKRCFREFCIPTSSTIEQQNSTRVRQNTREHPSTANTVDRTNHQLENLEAETAPLP398. To generate a specific amino acid mutation in the GST fusion protein of MOPr-CT, the GeneTailor™ Site-Directed Mutagenesis System kit (Invitrogen, Paisley UK) was used. All the recombinant pGEX-4T-1 plasmids were transformed into BL21 (DE3) E. coli competent cells. Bacteria were grown in LB broth and protein expression induced by 0.5 mM isopropyl-beta-D-thiogalactopyranoside (IPTG; Apollo Scientific, Stockport UK) at 15°C overnight.
Bacterial cells were harvested by centrifugation at 5,040 g and 4°C for 15 min. The cell pellets were then resuspended in a lysis buffer (20 mM HEPES pH 7.4, 100 mM KCl) supplemented with 1 mM EDTA, 20 mM β-mercaptoethanol, 1 mM dithiothreitol, and protease inhibitors (0.5 mM benzamidine hydrochloride, 4 μg/ml leupeptin, and 4 μg/ml pepstatin), followed by 4 periods of sonication (30 sec each on dial 3, Misonix sonicator XL2020) to disrupt the cells. Triton-X 100 (1 % final concentration) was added to sonicated cells, followed by a further incubation at 4°C with gentle shaking to ensure efficient cell lysis. The unwanted pellets were removed by centrifugation of the lysate at 43,100 g at 4°C for 20 min. The supernatant containing the GST-fusion proteins was further incubated at 4°C overnight, with a 50% slurry of glutathione sepharose 4B beads (GE Healthcare, Chalfont St Giles, UK), which had been prewashed and resuspended in lysis buffer. The GST-fusion protein-bound beads were then collected by a brief spin in a microcentrifuge, washed twice in lysis buffer, resuspended in 1 volume of lysis buffer and 2 volumes of glycerol and stored at -20°C.
Kinase-induced phosphorylation of GST fusion proteins
CaMKII requires activation by autophosphorylation before being used in the phosphorylation reaction. The activation reaction contained 1 μl (500 units) of CaMKII (New England BioLabs, Hitchin, UK) in the presence of 20 μM ATP, 1 mM calcium chloride, and 0.075 μM calmodulin in a final volume of 10 μl. Activation of the CaMKII mixture was performed at 30°C for 10 min to enable the autophosphorylation and hence activation of CaMKII.
The GST-fusion proteins immobilized on glutathione sepharose beads were washed and resuspended in assay buffer (20 mM HEPES pH7.4, 1 mM dithiothreitol) up to a final volume of 10 μl. The CaMKII-induced phosphorylation was initiated by incubating 5 μl of sample proteins in the presence of 10 μl of pre-activated CaMKII mixture, 5 μl of ATP mixture (0.02 mM ATP, 10 mM MgCl2), and 2.8 μCi [γ-32P]ATP to a final volume of 25 μl. The final concentration of CaMKII was 100 nM. The phosphorylation reaction was allowed to proceed at 30°C for 15 min.
For PKC-induced phosphorylation, 5 μl washed beads were incubated with PKC (Calbiochem, Nottingham Uk, final concentration 100 nM), 5 μl ATP mixture, and 2.8 μCi [γ-32P]ATP, in a final volume of 25 μl at 30°C for 15 min.
For GRK2-induced phosphorylation, the washed beads were resuspended in 20 mM HEPES pH7.4, 1 mM dithiothreitol, and 1 mM EDTA. Reaction mixtures contained 5 μl sample protein, 100 nM GRK2 , 5 μl of ATP mixture, and 2.8 μCi of [γ-32P]ATP in a total volume of 25 μl and were incubated at 30°C for 15 min.
The kinase-induced phosphorylation reactions were stopped by the addition of 5 x SDS sample buffer to the reactions and heating at 100°C for 5 min. The proteins were then resolved by 12 % SDS-PAGE. The protein bands were visualized by staining with Coomassie blue; the gels with phosphorylated bands were visualised by autoradiography.
The software Image J (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997-2009) was used to quantify the density of the phosphorylated bands on the autoradiograph, and the protein bands on the Coomassie blue-stained gel. The level of phosphorylation was quantified by normalizing the density of phosphorylated bands to the density of their corresponding protein bands, which represented the amount of protein used in each experiment. If no phosphorylated band was evident on the autoradiograph, the position corresponding to the protein band on the gel was identified and used in quantification. In the assays to identify phosphorylation sites for each kinase, after quantification as above, the phosphorylation level of each fusion protein containing the mutant MOPr sequence was compared to the phosphorylation level of the corresponding fusion protein containing WT sequence (taken as 100%).
Arrestin interaction with GST fusion proteins
Arrestin-2 and -3 were purified as previously described (Gurevich and Benovic, 2000; Zhan et al., 2011). GST fusion proteins on sepharose beads were made up to a total volume of 500 μl in assay buffer (20 mM Tris, pH 7.5, 200 mM NaCl), and different amounts of purified arrestin-2 or arrestin-3 (final concentrations of 40, 200, 500, 1000, 1500 and 2000 ng/ml; 2000 ng/ml is approximately equal to 44 nM) were added, and mixed by rotation at 4°C different lengths of time (30 min, 1 h or 2.5 h). Following the incubation, beads were recovered by centrifugation and washed three times with assay buffer and analysed by SDS-PAGE. For Western blotting, proteins were transferred onto polyvinylidene fluoride membrane and the membrane stained with Ponceau S to determine the amount of GST-fusion protein present. Following this the amount of arrestin-2 or -3 bound was determined using a rabbit polyclonal pan-arrestin antibody (1:3,000 dilution; Abcam, Cambridge, UK) and a donkey anti-rabbit IgG horseradish peroxidase (HRP)-linked secondary antibody (1:12,000 dilution; GE Healthcare). Amounts of GST-fusion protein and arrestin-2 or -3 present on membranes, were quantified using Image J.
Statistics
An unpaired t test was further used to compare the phosphorylation of GST fusion proteins to that of GST alone. A one-sample t test was used to compare the phosphorylation of mutants to the wild type GST fusion proteins, and to compare the binding of arrestins to different GST fusion proteins, and to phosphorylated and non-phosphorylated fusion proteins. Differences were regarded as significant when p < 0.05.
Results
Identification of phosphorylation sites in the C-terminal tail of MOPr in intact cells
When MOPr was affinity purified from HEK293 cells and subjected to SDS-PAGE, a band at the expected molecular weight for the rat MOPr (~ 80kDa) was observed (Fig. 1B). Mass spectrometric analysis of the purified MOPr identifed a series of peptide derived from the intracellular C-terminal tail. MS/MS sequencing of these peptides provided coverage of the entire C-terminal tail and identified a number of phospho-acceptor sites (Fig. 2 and see Supplementary Fig. 1). To assess the selective phosphorylation on the C-terminal tail of MOPr under basal and agonist-stimulated conditions, the HEK293 cells stably expressing HA-MOPr were incubated in the absence or presence of saturating concentrations of DAMGO (10 μM) or morphine (30 μM) for 10 min. Under basal conditions, Ser363 and Thr370 were found to be phosphorylated. In replicate 1, a singly phosphorylated peptide corresponding to the sequence around Ser363 was present but it was not possible to distinguish whether it was phosphorylated on Ser363 or Thr364. However, the MS/MS spectra generated in replicate 2 clearly showed the site to be Ser363.
On stimulation of the receptor with either morphine or DAMGO, these residues were still phosphorylated and three additional phosphorylation sites were found on Ser356, Thr357 and Ser375 (Fig. 2A). A summary of phosphorylated residues in the C-terminal tail of MOPr is shown in Fig. 2B.
GRK2 and second messenger-dependent protein kinases phosphorylate distinct residues on the C-terminal tail of MOPr expressed as a GST fusion protein
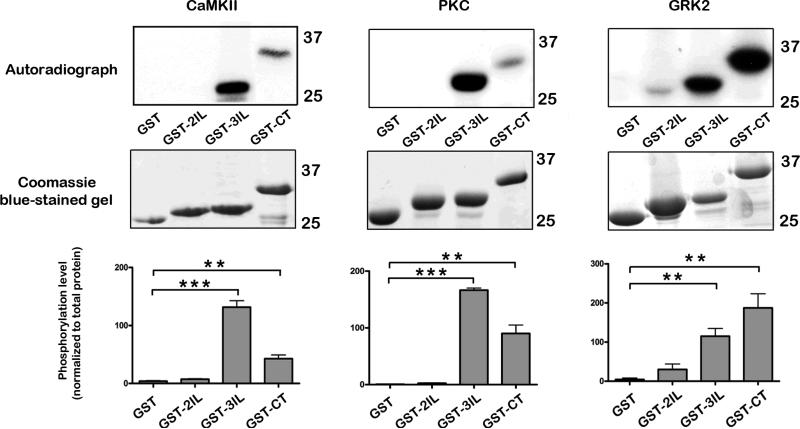
We investigated the in vitro ability of CaMKII, PKC and GRK2 to phosphorylate GST fusion proteins of the MOPr C-terminal tail in vitro using [γ-32P]-ATP incorporation. In preliminary experiments, we tested the ability of these kinases to phosphorylate the 2nd and 3rd intracellular loops of MOPr in comparison to the C-terminal tail. All three kinases were able to phosphorylate intracellular regions of MOPr expressed as GST-fusion proteins, whilst GST alone was not phosphorylated by any of the kinases (Fig. 3). Notably the GST-3IL was markedly phosphorylated by each kinase, whilst phosphorylation of the GST-CT was also observed with the kinases, with GRK2 being particularly effective in phosphorylating this region (Fig. 3). The specificity of CaMKII- and PKC-induced phosphorylation was confirmed with the selective inhibitors KN93 (10 μM) or GF109203X (10 μM), respectively (Supplementary Fig. 2A and B).
Fig. 3.
In vitro phosphorylation of intracellular regions of MOPr. GST fusion proteins (GST alone, GST-2IL, GST-3IL, GST-CT) immobilized on glutathione-Sepharose beads were incubated with purified CaMKII (preactivated), PKC, or GRK2 in the presence of [γ32P]ATP. Following SDS-PAGE, kinase-induced phosphorylation was detected by autoradiography (top panels), whilst the relative amounts of GST-fusion proteins were determined with Coomassie blue (middle panels). Bar graphs showing quantitation of phosphorylation are shown (bottom panels). Values are means ± SEM from 3-4 independent experiments in each case. All three kinases produced significant phosphorylation of the 3rdIL and CT of MOPr (unpaired t test, *** p < 0.001, ** p < 0.01 compared to GST alone).
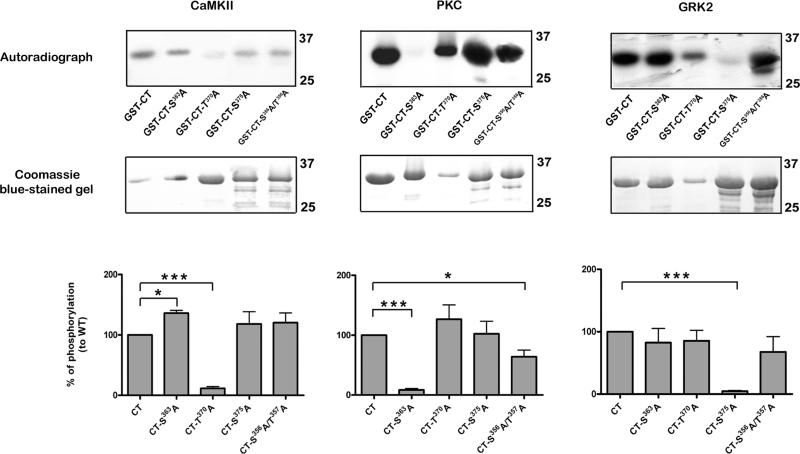
Further experiments were directed at identifying the phosphorylated residues in the COOH terminal tail of MOPr, since this region is mostly associated with receptor regulation (Kelly 2011). Point mutations were introduced into GST fusion proteins at residues likely to undergo phosphorylation. These experiments (Fig. 4) indicated clearly that, under these in vitro conditions, Thr370 is the main substrate for CaMKII phosphorylation, Ser363 the main substrate for PKC phosphorylation, and Ser375 the main substrate for GRK2 phosphorylation. In addition, CaMKII-induced phosphorylation of the Ser363Ala mutant was moderately but significantly increased, whilst PKC-induced phosphorylation of the Ser356Ala/Ser357Ala double mutant was moderately but significantly reduced.
Fig. 4.
Identification of In vitro phosphorylation sites in the C-terminal tail of MOPr. GST fusion proteins (wild type and mutant) immobilized on glutathione-Sepharose beads were incubated with purified CaMKII (preactivated), PKC, or GRK2 in the presence of [γ32P]ATP. Following SDS-PAGE, kinase-induced phosphorylation was detected by autoradiography (top panels), whilst the relative amounts of GST-fusion proteins were determined with Coomassie blue (middle panels). Bar graphs showing quantitation of phosphorylation relative to wild type (CT) constructs are shown (bottom panels). Values are means ± SEM from 3-4 independent experiments in each case. CaMKII phosphorylation was significantly reduced in the Thr370Ala mutant, PKC phosphorylation in the Ser363Ala mutant, and GRK2 phosphorylation in the Ser375Ala mutant (one sample t test, *** p< 0.001 compared to respective wild type). PKC phosphorylation was also reduced in the Ser356Ala/Thr357Ala double mutant (* p< 0.01 compared to respective wild type), whilst CaMKII phosphorylation was increased in the Ser363Ala mutant (* p< 0.01 compared to respective wild type).
Arrestin-2 and -3 binding to the intracellular regions of MOPr
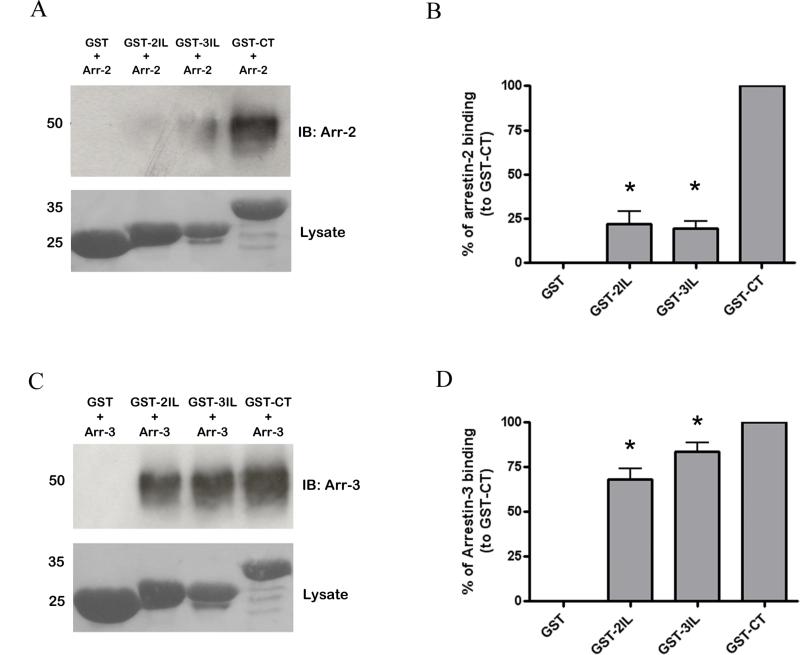
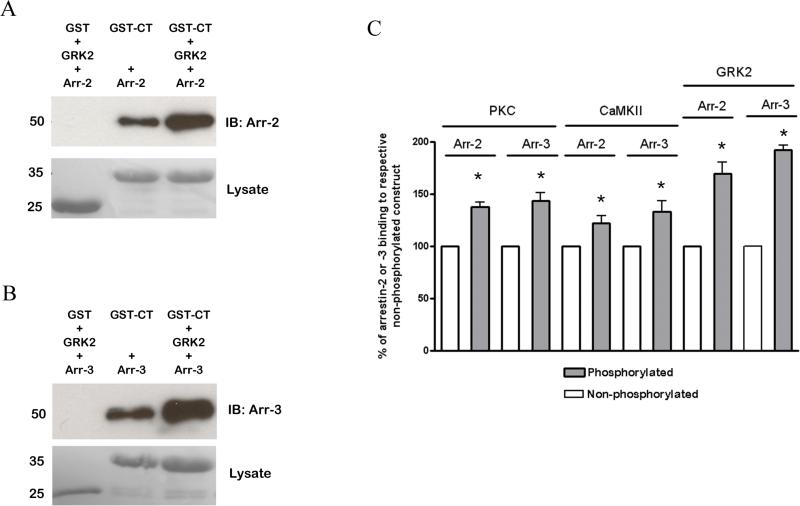
The ability of arrestin-2 and -3 to bind to GST fusion proteins of the intracellular regions of MOPr in vitro was also examined. In preliminary experiments, the interaction of purified arrestin-2 or -3 with GST-CT was shown to be concentration- and time-dependent (Supplementary Fig. 3 and 4), the latter being similar to that previously reported for arrestin interaction with other GPCRs (Gurevich et al., 1995). The ability of purified arrestins to interact with the intracellular regions of MOPr was then tested. Interestingly, whereas arrestin-3 bound avidly to GST-2IL and GST-3IL as well as GST-CT (Fig. 5C and D), arrestin-2 interacted much more readily with the C-terminal tail fusion protein than with those of the intracellular loops (Fig. 5A and B). We also tested the ability of GST-CT to interact with arrestins following phosphorylation by GRK2, PKC or CaMKII. The fusion protein was phosphorylated by the kinases as described above. Following this in vitro phosphorylation, the GST fusion protein was incubated with arrestin-2 or arrestin-3. Under these conditions phosphorylation by each of the three kinases enhanced the ability of arrestin-2 and -3 to bind to the GST fusion protein of MOPr, with the greatest increase in interaction being seen following GRK2 phosphorylation (Fig. 6).
Fig. 5.
Arrestin-2 and -3 interaction with GST fusion proteins of the intracellular regions of MOPr. Purified (A, B) arrestin-2 or (C, D) arrestin-3 (final concentration 500 ng/ml in each case, approximately 11 nM) was incubated with GST alone or with GST-2IL, GST-3IL or GST-CT. Example blots are shown in (A) and (C), whilst bar graps of data from 3-4 experiments are shown in (B) and (D). These results are shown as a % of arrestin binding to the GSTCT, taken as 100%, with error bars representing S.E.M. * p < 0.05 compared to binding of relevant arrestin to GST-CT, one-sample t test.
Fig. 6.
Phosphorylation increases the interaction of arrestin-2 and -3 with the GST-fusion protein of the C-terminal tail of MOPr. The GST-CT was subjected to phosphorylation by CaMKII, PKC, or GRK2 as described in the Methods section and their ability to interact with arrestin-2 or -3 (final concentration 500 ng/ml in each case, approximately 11 nM) was compared with that for nonphosphorylated GST-CT. Representative blots of the interaction of arrestin-2 (A) and arrestin-3 (B) with the non-phosphorylated and GRK2-phosphorylated GST-CT are shown, as well as (C) a bar graph showing data from at least 4 independent experiments. In all cases, phosphorylation increased arrestin-2 and arrestin-3 binding to the GST-CT. These results are shown as a % of arrestin binding to the non-phosphorylated GST-CT, taken as 100%, with error bars representing S.E.M. * p < 0.05 compared to binding of relevant arrestin to non-phosphorylated GST-CT (taken in each case as 100%), one-sample t test.
Discussion
These results provide unequivocal identification of phosphorylated residues in the C-terminal tail of MOPr expressed in HEK293 cells. The key findings of this work are that in these cells MOPr is phosphorylated in the absence of agonist; that DAMGO- and morphine-induced phosphorylation involves multiple but similar residues in the C-terminal tail; that in vitro three kinases, CaMKII, PKC, and GRK2 phosphorylate distinct residues in the C-terminal tail of MOPr; that in vitro arrestin-2 and -3 interact with the C-terminal tail of MOPr, and that this interaction is increased following kinase-induced phosphorylation of the protein.
A cluster of four amino acids (Thr354Ser355Ser356Thr357; refer to Fig. 2B) in the C-terminal tail of MOPr was previously reported to mediate agonist-induced desensitization of receptor responsiveness (Wang 2000), with Ser355 and Thr357 in particular being identified as likely phosphoacceptor sites (Wang et al., 2002). However others have reported that this cluster is not involved in either agonist-induced phosphorylation or desensitization of MOPr (Deng et al. 2000; El Kouhen et al. 2001). Here we confirm that Thr357 is phosphorylated in response to agonist and also identify Ser356 as a phosphorylated residue, rather than Ser355 as suggested previously (Wang et al. 2002). Furthermore our in vitro 32P-incorporation experiments identify PKC as a possible kinase for one or both of these sites, however it is also possible that mutation on this Ser/Thr pair instead affects the PKC-induced phosphorylation of the nearby Ser363.
On the basis of point mutations, Ser363 and Thr370 in the C-terminal tail of MOPr were suggested to be phosphoacceptor sites (El Kouhen et al. 2001). The present results using mass spectrometry confirm this and, in addition, demonstrate that these residues can be phosphorylated in the absence of agonist treatment. However this does not preclude the possibility that these residues are further phosphorylated in the presence of agonist. A recent study (Doll et al. 2011) with antiphosphoreceptor antibodies also identifies Ser363 and Thr370 as phosphoacceptor residues, although these authors concluded that Thr370 was only phosphorylated in response to DAMGO, and not with morphine or under basal conditions. Another recent study (Feng et al. 2011) indicated that phorbol ester-induced desensitization of MOPr is mediated by PKC-dependent phosphorylation of Ser363. Interestingly using the in vitro GST fusion protein 32P-incorporation assay, we now show that Ser363 and Thr370 are preferentially phosphorylated by PKC and CaMKII, respectively. MOPr has been suggested to be pre-phosphorylated by PKC (Johnson et al. 2006), which may underlie the ability of morphine to induce MOPr desensitization in neurones (Bailey et al. 2009a; Bailey et al. 2009b). In addition CaMKII activation has been implicated in morphine tolerance (Mestek et al. 1995; Koch et al. 1997; Fan et al. 1999). It remains to be seen whether or not such effects are indeed due to phosphorylation of these residues on MOPr by these second messenger-dependent kinases, or instead due to phosphorylation of other cellular substrates that contribute to morphine tolerance.
An anti-phosphoserine375 antibody has been developed and its use has already demonstrated that Ser375 is phosphorylated in an agonist-dependent manner (Schulz et al. 2004; Chu et al. 2008; McPherson et al. 2010). We confirm here that this residue is indeed phosphorylated in response to agonist. Mutation of this Ser375 has been reported to inhibit agonist-induced desensitization (Ozsoy et al. 2005; Chu et al. 2008) and internalization of MOPr (El Kouhen et al. 2001), although in another study this mutation inhibited morphine- but not DAMGO-induced desensitization (Schulz et al. 2004), whilst in yet other studies MOPr constructs with multiple mutations including of Ser375 were still able to undergo agonist-induced desensitization similar to the wild type MOPr (Pak et al. 1997; Deng et al. 2000). Clearly the precise role of Ser375 and its phosphorylation in MOPr function requires further study. On the other hand, Ser375 has been suggested to be the phosphorylation site for GRK2 (Schulz et al., 2004) and in vitro phosphorylation data from the present study suggests that GRK2 is the likely kinase involved, although other GRKs may also be involved in vivo. In addition the ability of GRK2 to phosphorylate the agonist-occupied receptor is likely to be much greater than its ability to phosphorylate receptor fusion protein sequences (Benovic et al., 1990). A recent study identifies further phosphoacceptor sites in a cluster (S375TANT) around Ser375 including Thr376 and Thr379 (Lau et al. 2011; Moulédous et al. 2012); phosphorylation of these residues was associated with arrestin interaction with MOPr (Lau et al. 2011). However with regard to the data in the present study, it will be interesting to assess the effect of mutating the residues whose phosphorylation is agonist-dependent (Ser356, Thr357 and Ser375), on agonist-induced desensitization and trafficking of MOPr.
On the basis of mutation data Thr394 has been identified in more than one study as a possible phosphoacceptor site (Pak et al. 1997; Deng et al. 2000). We were unable to detect phosphorylation of this residue, strongly suggesting that Thr394 is not phosphorylated. However with our present data we cannot absolutely rule out Thr394 as a phosphoacceptor site since it remains theoretically possible that the relevant phosphopeptide was missed in our analysis, but it is noteworthy that phosphorylation in the distal section of the COOH-terminus of MOPr was not detected in other recent studies (Lau et al. 2011; Moulédous et al. 2012). Mutation of Thr394 has also been reported to markedly reduce agonist-induced desensitization and down regulation of MOPr (Pak et al. 1997; Deng et al. 2000), whilst a mutant MOPr with Thr394 mutated to alanine displays increased internalization and resensitization (Wolf et al. 1999). However, if Thr394 is not phosphorylated, then it is possible that this residue or the region of the COOH-terminus around it has some wider role in controlling MOPr function, such as a permissive role in kinase recruitment or perhaps this region of MOPr interacts with scaffolding proteins that are important for appropriate receptor regulation. The latter is supported by the finding that mutation of three glutamate residues close to Thr394 also inhibited MOPr desensitization (Pak et al. 1997).
With regard to the GST-fusion protein phosphorylation data in this study, it should be noted that the phosphorylation of a peptide by a particular kinase may not always reflect events in the intact cell. In addition the potential interaction of the C-terminal tail of the intact MOPr with other intracellular regions of the receptor, or with interacting proteins or even the membrane itself could influence the nature and extent of phosphorylation. These potential influences would be absent in the GST-fusion protein experiments. Furthermore, there is the question of the cellular activation of the three kinases identified as mediating the phosphorylation of the C-terminal tail. Whilst there is clear evidence that following MOPr activation, GRK (Doll et al. 2012) and PKC (Chu et al. 2010) are activated, there is less evidence to implicate CaMKII (Mestek et al. 1995, Sanchez-Blazquez et al. 2008), although in the intact brain these kinases could be activated by ongoing neuronal activity and consequent increases in intracellular free Ca2+; such a mechanism would give rise to heterologous regulation of MOPr. Furthermore the present results report phosphorylation of MOPr expressed in HEK293 cells, but it is possible that the receptor phosphorylation profile would be different in another cellular context such as neurones (Tobin et al. 2008; Torrecilla et al. 2007). Nevertheless it is of interest that morphine-induced desensitization of MOPr in the locus coeruleus requires ongoing PKC activity (Bailey et al. 2009a; Bailey et al. 2009b), suggestive of the possibility that basal MOPr phosphorylation also occurs in neurones. It is also possible that MOPr is phosphorylated at intracellular sites in addition to the C-terminal tail; for example Thr180 in the third intracellular loop of MOPr has been suggested to be a GRK phosphorylation site (Celver et al., 2001).
Previous studies have suggested that non-visual arrestins interact poorly if at all with the intracellular regions of MOPr (Cheng et al. 1998; Cen et al. 2001), although our recent work indicates that arrestin-3 does associate with intact MOPr in intact cells in an agonist-dependent manner (McPherson et al. 2010). In the present study we found a robust interaction between purified arrestins and GST fusion proteins, with both arrestin-2 and -3 being able to associate with the GST fusion protein of the C-terminal tail of MOPr. Interestingly we found that phosphorylation of the GST-CT increased its interaction with each arrestin. Under the conditions of the assay GRK2 phosphorylation produced the greatest increase in arrestin association. Whether or not phosphorylation of Ser363 or Thr370 affect arrestin association or MOPr trafficking in intact cells is less clear, but it should be noted that Ser363 is basally phosphorylated but does not lead to constitutive arrestin association with the receptor (Zhang et al., 1998; McPherson et al., 2010). Indeed, mutation of Ser363 actually enhances agonist-induced internalization of MOPr (El Kouhen et al. 2001). On the other hand previous studies have shown that GPCRs normally require phosphorylation of two or more residues for tight binding of arrestins to occur (Gurevich et al., 1995; Vishnivetskiy et al., 2007), perhaps explaining the inability of arrestin to associate with the basally-phosphorylated MOPr in intact cells. Recent work also indicates that dependency on GPCR phosphorylation versus receptor activation for arrestin association is receptor subtype-dependent (Gimenez et al. 2012). The present results and those from other studies (Lowe et al., 2002; Lau et al. 2011; see references in Gurevich and Gurevich, 2006) suggest that arrestin association with the C-terminal tail of MOPr as well as other GPCRs has both phosphorylation-dependent and phosphorylation-independent components.
In conclusion, we have identified phosphorylated residues in the C-terminal tail of the rat MOPr stably expressed in HEK293 cells by LC-MS/MS. We have also identified candidate kinases for the phosphorylation of some of these residues, and show that phosphorylation by a range of protein kinases can enhance the association of arrestins with MOPr. Together these findings support the idea that MOPr signalling and regulation can be modulated by multiple kinase-dependent mechanisms.
Supplementary Material
Acknowledgements
The authors have no conflict of interest to declare. This work was supported by a project grant to EK from the Medical Research Council UK, and NIH grant GM077561 to VVG. ABT and AJB are supported by the Medical Research Council UK. The proteomic mass spectrometry experiments were conducted by the Protein and Nucleic Acid Chemistry Laboratory (PNACL) at Leicester University.
Abbreviations used
- MOPr
μ opioid receptor
- DAMGO
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor
- HA
hemagglutinin
- LC-MS/MS
Liquid Chromatography with Tandem Mass Spectoscopy
- LTQ
Linear Trap Quadrupole
- MOPr
μ opioid receptor
Footnotes
Note: The LC-MS/MS data described here was first presented as an abstract at the July 2010 INRC in Malmo, Sweden (http://www.inrcworld.org/2010/INRC%20program&abstract%20Final.pdf) page 99.
References
- Bailey C, Llorente J, Gabra B, Smith F, Dewey W, Kelly E, Henderson G. Role of protein kinase C and μ-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur. J. Neurosci. 2009a;29:307–18. doi: 10.1111/j.1460-9568.2008.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, Henderson G. Involvement of PKCα and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of μ-opioid receptors in mature brain neurons. Brit. J. Pharmacol. 2009;158:157–64. doi: 10.1111/j.1476-5381.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic JL, Onorato J, Lohse MJ, Dohlman HG, Staniszewski C, Caron MG, Lefkowitz RJ. Synthetic peptides of the hamster beta 2-adrenoceptor as substrates and inhibitors of the beta-adrenoceptor kinase. Brit. J. Clin. Pharmacol. 1990;30(Suppl 1):3S–12S. doi: 10.1111/j.1365-2125.1990.tb05462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd A, El-Kouhen R, Erickson L, Loh H, Law P. Identification of serine 356 and serine 363 as the amino acids involved in etorphine-induced down-regulation of the μ-opioid receptor. J. Biol. Chem. 1998;273:34488–95. doi: 10.1074/jbc.273.51.34488. [DOI] [PubMed] [Google Scholar]
- Busillo J, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic J. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 2010;285:7805–17. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celver JP, Lowe J, Kovoor A, Gurevich VV, Chavkin C. Threonine 180 is required for GRK3 and arrestin3 mediated desensitization of Mu opioid receptor in Xenopus oocytes. J. Biol. Chem. 2001;276:4894–4900. doi: 10.1074/jbc.M007437200. [DOI] [PubMed] [Google Scholar]
- Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol. Pharmacol. 2001;59:758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Yu QM, Wu YL, Ma L, Pei G. Selective interference of beta-arrestin 1 with kappa and delta but not mu opioid receptor/G protein coupling. J. Biol. Chem. 1998;273:24328–24333. doi: 10.1074/jbc.273.38.24328. [DOI] [PubMed] [Google Scholar]
- Christoffers K, Li H, Keenan S, Howells R. Purification and mass spectrometric analysis of the μ opioid receptor. Brain Res. Mol. Brain Res. 2003;118:119–31. doi: 10.1016/j.molbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Chu J, Zheng H, Loh H, Law P. Morphine-induced μ-opioid receptor rapid desensitization is independent of receptor phosphorylation and β-arrestins. Cell. Signal. 2008;20:1616–24. doi: 10.1016/j.cellsig.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization. Cell Signal. 2010;22:684–696. doi: 10.1016/j.cellsig.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Brit. J. Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Yu Y, Pak Y, O'Dowd B, George S, Surratt C, Uhl G, Wang J. Role for the C-terminus in agonist-induced μ opioid receptor phosphorylation and desensitization. Biochemistry. 2000;39:5492–9. doi: 10.1021/bi991938b. [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. Agonist-selective patterns of mu-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Brit. J. Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Poll F, Peuker K, Loktev A, Gluck L, Schulz S. Deciphering μ-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br. J. Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.02080.x. doi: 10.1111/j.1476-5381.2012.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Burd A, Erickson-Herbrandson L, Chang C, Law P, Loh H. Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J. Biol. Chem. 2001;276:12774–80. doi: 10.1074/jbc.M009571200. [DOI] [PubMed] [Google Scholar]
- Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol. Pharmacol. 1999;56:39–45. doi: 10.1124/mol.56.1.39. [DOI] [PubMed] [Google Scholar]
- Feng B, Li Z, Wang JB. Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signalling. Mol. Pharmacol. 2011;79:768–75. doi: 10.1124/mol.110.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez LE, Kook S, Vishnivetskiy SA, Ahmed MR, Gurevich EV, Gurevich VV. Role of Receptor-attached Phosphates in Binding of Visual and Non-visual Arrestins to G Protein-coupled Receptors. J. Biol. Chem. 2012;287:9028–9040. doi: 10.1074/jbc.M111.311803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interactions with G protein-coupled receptors: Direct binding studies with rhodopsin, β2-adrenergic, and m2 muscarinic cholinergic receptors. J. Biol. Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Arrestin: mutagenesis, expression, purification, and functional characterization. In: Palczewski K, editor. Methods in Enzymology. Vol. 315. 2000. pp. 422–437. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Christie M, Connor M. The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals. 2005;14:290–302. doi: 10.1159/000093044. [DOI] [PubMed] [Google Scholar]
- Johnson E, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of μ-opioid receptor desensitization in human embryonic kidney 293 cells. Mol. Pharmacol. 2006;70:676–85. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- Kelly E. The subtleties of μ-opioid receptor phosphorylation. Brit. J. Pharmacol. 2011;164:294–297. doi: 10.1111/j.1476-5381.2011.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Brit. J. Pharmacol. 153 Suppl. 2008;1:S379–388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Braud S, Isaac J, Roche K. Protein kinase C phosphorylation of the metabotropic glutamate receptor mGluR5 on Serine 839 regulates Ca2+ oscillations. J. Biol. Chem. 2005;280:25409–15. doi: 10.1074/jbc.M502644200. [DOI] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J. Neurochem. 1997;69:1767–1770. doi: 10.1046/j.1471-4159.1997.69041767.x. [DOI] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, Von Zastrow M. Quantitative encoding of the effect of a partial agonist on individual opioids receptors by multisite phosphorylation and threshold detection. Sci. Signal. 2011;4(185):ra52. doi: 10.1126/scisignal.2001748. [DOI: 10.1126/scisignal.2001748] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe JD, Celver JP, Gurevich VV, Chavkin C. Mu opioid receptors desensitize less rapidly than delta opioid receptors due to less efficient activation of arrestin. J. Biol. Chem. 2002;277:15729–35. doi: 10.1074/jbc.M200612200. [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E. μ-Opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol. Pharmacol. 2010;78:756–66. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestek A, Hurley J, Bye L, Campbell A, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J. Neurosci. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouledous L, Froment C, Dauvillier S, Burlet-Schiltz O, Zajac JM, Mollereau C. GRK2-mediated trans-phosphorylation contributes to the loss of function of mu opioid receptors induced by neuropeptide FF (NPFF2) receptors. J. Biol. Chem. 2012;287:12736–49. doi: 10.1074/jbc.M111.314617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles KN, Xiao K, Ahn S, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy H, Thakker D, Standifer K. Orphanin FQ/nociceptin potentiates [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin-induced μ-opioid receptor phosphorylation. Mol. Pharmacol. 2005;68:447–56. doi: 10.1124/mol.105.011536. [DOI] [PubMed] [Google Scholar]
- Pak Y, O'Dowd B, George S. Agonist-induced desensitization of the μ opioid receptor is determined by threonine 394 preceded by acidic amino acids in the COOH-terminal tail. J. Biol. Chem. 1997;272:24961–5. doi: 10.1074/jbc.272.40.24961. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Rodriguez-Munoz M, Montero C, de la Torre-Madrid E, Garzon J. Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacol. 2008;54:319–330. doi: 10.1016/j.neuropharm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. Morphine induces terminal μ-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–9. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A, Butcher A, Kong K. Location, location, location...site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol. Sci. 2008;29:413–20. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla I, Spragg E, Poulin B, McWilliams P, Mistry S, Blaukat A, Tobin A. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J. Cell. Biol. 2007;177:127–37. doi: 10.1083/jcb.200610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the β-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–43. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Raman D, Wei J, Kennedy MJ, Hurley JB, Gurevich VV. Regulation of arrestin binding by rhodopsin phosphorylation level. J. Biol. Chem. 2007;282:32075–83. doi: 10.1074/jbc.M706057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. A cluster of Ser/Thr residues at the C-terminus of μ-opioid receptor is required for G protein-coupled receptor kinase 2-mediated desensitization. Neuropharmacology. 2000;39:353–63. doi: 10.1016/s0028-3908(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Chang W, Hsu C, Huang P, Chow Y, Li A. Identification of two C-terminal amino acids, Ser355 and Thr357, required for short-term homologous desensitization of mu-opioid receptors. Biochem. Pharmacol. 2002;64:257–66. doi: 10.1016/s0006-2952(02)01114-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Guang W, Barbier E, Shapiro P, Wang J. μ Opioid receptor mutant, T394A, abolishes opioid-mediated adenylyl cyclase superactivation. Neuroreport. 2007;18:1969–73. doi: 10.1097/WNR.0b013e3282f228b2. [DOI] [PubMed] [Google Scholar]
- Wannemacher K, Terskiy A, Bian S, Yadav P, Li H, Howells R. Purification and mass spectrometric analysis of the kappa opioid receptor. Brain Res. 2008;1230:13–26. doi: 10.1016/j.brainres.2008.06.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R, Koch T, Schulz S, Klutzny M, Schröder H, Raulf E, Bühling F, Höllt V. Replacement of threonine394 by alanine facilitates internalization and resensitization of the rat μ opioid receptor. Mol. Pharmacol. 1999;55:263–8. doi: 10.1124/mol.55.2.263. [DOI] [PubMed] [Google Scholar]
- Wu S, Birnbaumer M, Guan Z. Phosphorylation analysis of G protein-coupled receptor by mass spectrometry: identification of a phosphorylation site in V2 vasopressin receptor. Anal. Chem. 2008;80:6034–7. doi: 10.1021/ac8008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc. Natl. Acad. Sci. U S A. 1998;95:7157–62. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual subtypes. J. Mol. Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.