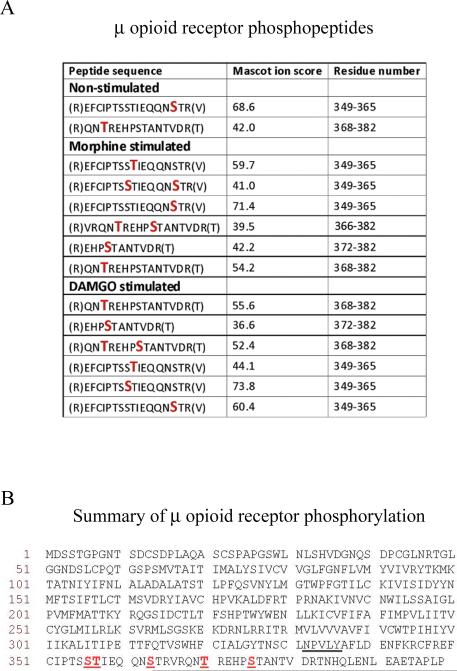
Fig. 2.
Identity of phosphorylated residues in MOPr. (A) MOPr phosphopeptides obtained in the presence or absence of morphine or DAMGO. Phosphorylated Ser or Thr residues are denoted by larger red letters. Spectra were analysed against the UniProtKB/SwissProt database using MASCOT software with peptide tolerance set to 5 ppm and the MS/MS tolerance set to 0.6 Daltons. The spectra of peptides reported as being phosphorylated were interrogated manually to confirm the precise sites of phosphorylation. (B) Summary of phosphorylation sites in the MOPr C-terminal tail. The phosphorylated Ser or Thr residues (Ser356, Thr357, Ser363, Thr370, and Ser375) are underlined, as is the NPXXY motif, at the base of the 7th transmembrane domain where the C-terminus begins. The mass spectrometry experiments described here were carried out three times for each condition. The data presented is a summary of these experiments and the spectra shown in Supplementary Fig. 1 are representative of the results obtained.