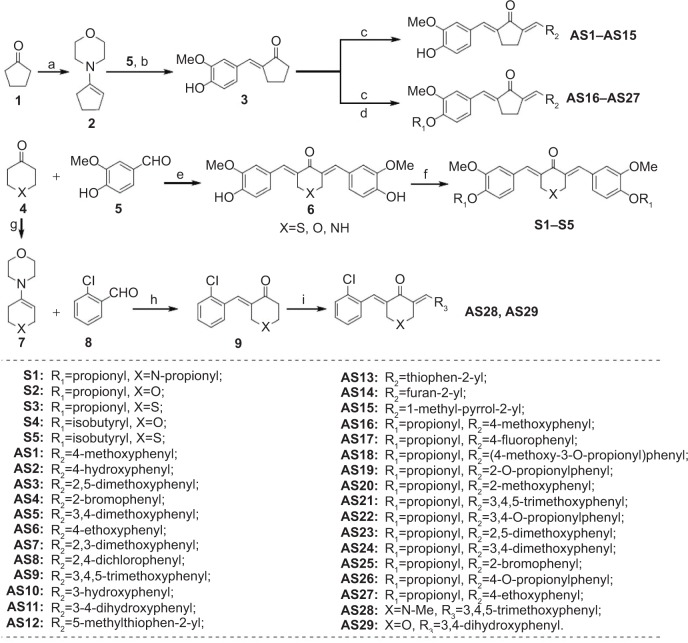
Figure 2.
The synthetic pathway for monocarbonyl curcumin analogs S1–S5, and AS1–AS29.
Note: Reagents and conditions were a) morpholine, p-TSA, cyclohexane, reflux, 50%; b) vanillin, EtOH, reflux, HCl, 30%; c) differently aldehydes, HCl gas, EtOH, 50°C–70°C, 10%–70%; d) THF, propionyl chloride, Et3N, rt, 5%–54%; e) piperidone, pyrone, or thiopyrone, HCl gas, EtOH, rt, 40%–50%; f) THF, propionyl, or isobutyryl chloride, Et3N, rt, 8%–31%; g) piperidone or pyrone, p-TSA, cyclohexane, reflux, 40%–50%; h) 2-chlorodebenzaldehyde, EtOH, reflux, HCl, 30%–35%; i) 3,4-dihydroxybenzaldehyde or 3,4,5-trimethoxybenzaldehyde, HCl gas, EtOH, rt, 10%–15%.
Abbreviations: AS, asymmetrical monocarbonyl curcumin derivative; EtOH, ethanol; Et3N, triethylamine; Me, methyl; rt, room temperature; THF, tetrahydrofuran; TSA, toluenesulfonic acid; HCl, hydrochloric acid.