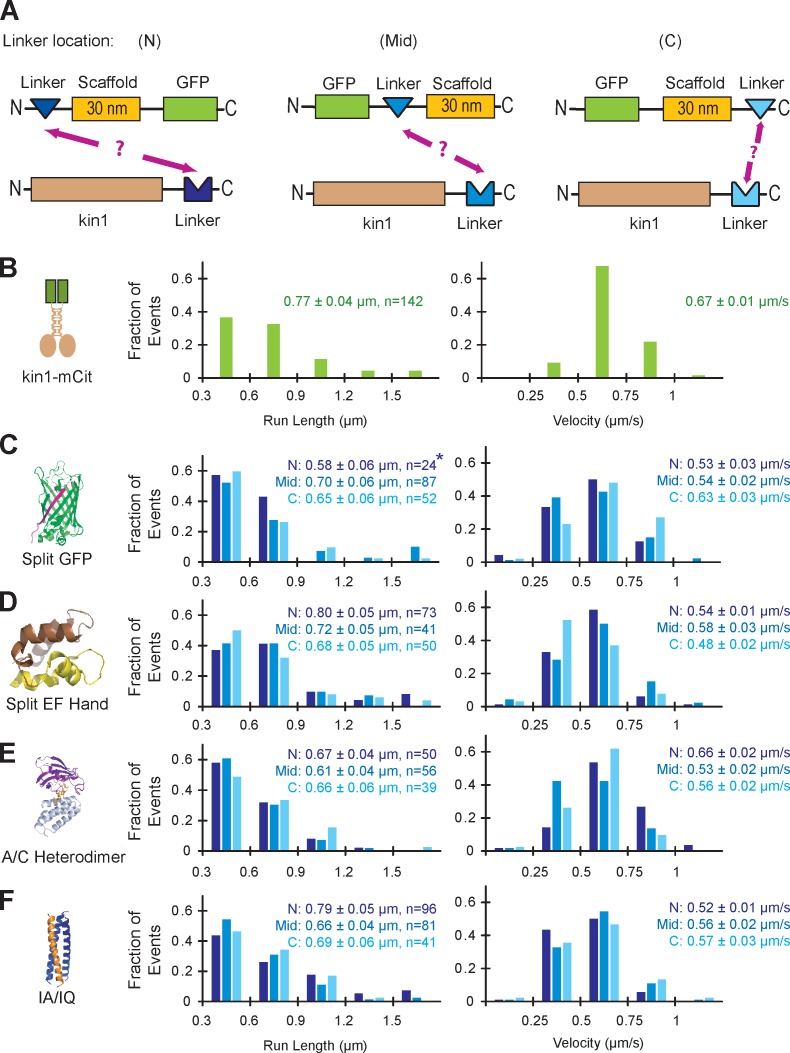
Figure 2.
Characterization of self-assembling linker components. (A) The ability of each linker to connect a nonfluorescent kin1 motor to a GFP-labeled SAH scaffold was tested for linkers positioned at the N terminus (N), middle (Mid), or C terminus (C) of the scaffold. (B–F) Single molecule motility assays. Motor–linker and linker–scaffold–GFP components were coexpressed in COS7 cells, and motility was analyzed in cell lysates. The run lengths (left) and velocities (right) of each population were plotted as histograms and the mean ± standard error (SE) is indicated. (B) The motility of mCitrine (mCit)-labeled kin1 motors (n = 142 events) was recorded as a positive control. (C) For the split GFP linker, strands 1–10 of the GFP barrel (GFP1–10) were placed at N, Mid, and C locations (n = 24, 87, and 52 events, respectively), and strand 11 (GFP11) was fused to the C terminus of kin1. The asterisk indicates poor expression of the scaffold in COS7 cells; see Fig. S1 B. (D) For the split EF Hand linker, the N-terminal half of the EF Hand was placed at the N (n = 73) or Mid (n = 41) locations and the C-terminal half of the EF Hand was placed at the C (n = 50) location. No self-assembly was observed for the N-terminal half of the EF Hand at the C location or for the C-terminal half of the EF Hand at the N location (not depicted). (E) For the A/C Heterodimer, DmrA (FKBP) was placed at the N, Mid, and C locations (n = 50, 56, and 39 events, respectively), whereas DmrC (FRB) was fused to kin1. (F) For the IA/IQ heterotrimeric coiled coil, the IQ sequence was placed at the N, Mid, or C locations (n = 96, 81, and 41 events, respectively), and the IA sequence was fused to kin1.