Abstract
Purpose
This prospective phase II study was designed to assess disease control and to describe acute and late adverse effects of treatment with proton radiotherapy in children with rhabdomyosarcoma (RMS).
Patients and Methods
Fifty-seven patients with localized RMS (age 21 years or younger) or metastatic embryonal RMS (age 2 to 10 years) were enrolled between February 2005 and August 2012. All patients were treated with chemotherapy based on either vincristine, actinomycin, and cyclophosphamide or vincristine, actinomycin, and ifosfamide–based chemotherapy and proton radiation. Surgical resection was based on tumor site and accessibility. Common Terminology Criteria for Adverse Events, Version 3.0, was used to assess and grade adverse effects of treatment. Concurrent enrollment onto Children's Oncology Group or European Pediatric Sarcoma Study Group protocols was allowed. All pathology and imaging were reviewed at the treating institution.
Results
Median follow-up was 47 months (range, 14 to 102 months) for survivors. Five-year event-free survival (EFS), overall survival (OS), and local control (LC) were 69%, 78%, and 81%, respectively, for the entire cohort. The 5-year LC by risk group was 93% for low-risk and 77% for intermediate-risk disease. There were 13 patients with grade 3 acute toxicity and three patients with grade 3 late toxicity. There were no acute or late toxicities higher than grade 3.
Conclusion
Five-year LC, EFS, and OS rates were similar to those observed in comparable trials that used photon radiation. Acute and late toxicity rates were favorable. Proton radiation appears to represent a safe and effective radiation modality for pediatric RMS.
INTRODUCTION
There are approximately 350 cases of rhabdomyosarcoma (RMS) per year in the United States, accounting for 3.8% of solid malignancies in children younger than age 19 years.1 Combined-modality therapy leads to cure in most children with localized disease, resulting in more than 70% surviving 5 years after diagnosis.2,3 Radiation therapy (RT) is an important component of treatment in many of these patients.4–7 However, RT in the pediatric population can be associated with both short- and long-term morbidity, depending on the volume treated and the dose delivered.
Proton RT can decrease normal tissue doses by a factor of 2 to 3 and therefore continues to hold potential in reducing the toxicity of treatment in the pediatric population.8,9 Dosimetric studies of proton therapy versus intensity-modulated radiation therapy (IMRT) in RMS have demonstrated greater sparing of ipsilateral and contralateral critical structures in both head and neck and genitourinary sites.10–13
When this trial opened in 2004, there were only three proton centers in the United States (currently, there are 14 open centers) and almost no clinical outcome data aside from disease control rates reported in the literature. To date, dosimetric studies and small patient cohorts still dominate the literature. In an effort to more clearly describe the rates of disease control and collect health outcomes, including adverse effects attributable to treatment, we embarked on a multi-institutional phase II study. This study, supported by the National Institutes of Health, represents the first prospectively collected health outcome data from a large cohort of pediatric patients with RMS treated with proton therapy.
PATIENTS AND METHODS
Patients
The study opened at Massachusetts General Hospital on October 31, 2004, and at the MD Anderson Cancer Center on March 26, 2010. Eligible patients included children with low- or intermediate-risk RMS, as defined by the accruing Children's Oncology Group (COG) protocols at the start of this phase II study. Specifically, this included children younger than age 22 years with newly diagnosed localized RMS or patients with metastatic embryonal RMS if their age was between 2 and 10 years. Pathology and imaging were reviewed at the treating proton therapy institution. Before enrollment, each patient underwent a complete medical evaluation and staging. Follow-up for study purposes included weekly treatment visits during RT, a visit 6 to 12 weeks after completing RT, and yearly visits thereafter with appropriate imaging. In the event that the patient was not local and was unable to return to the treating proton center for follow-up, imaging and detailed medical records from the referring institution were obtained.
Treatment
Patients were required to receive a chemotherapy regimen accepted as standard for RMS, and concurrent enrollment on North American and European protocols was allowed. All patients received chemotherapy based on either vincristine, actinomycin, and cyclophosphamide or vincristine, actinomycin, and ifosfamide and passively scattered proton RT. Surgical resection was used when clinically appropriate. RT dose depended on the extent of residual tumor and primary site of disease, and it typically conformed to the current or recently closed COG protocols. The timing of RT was determined by the COG protocol the patient was enrolled onto or treated according to or in consultation with the referring doctors.
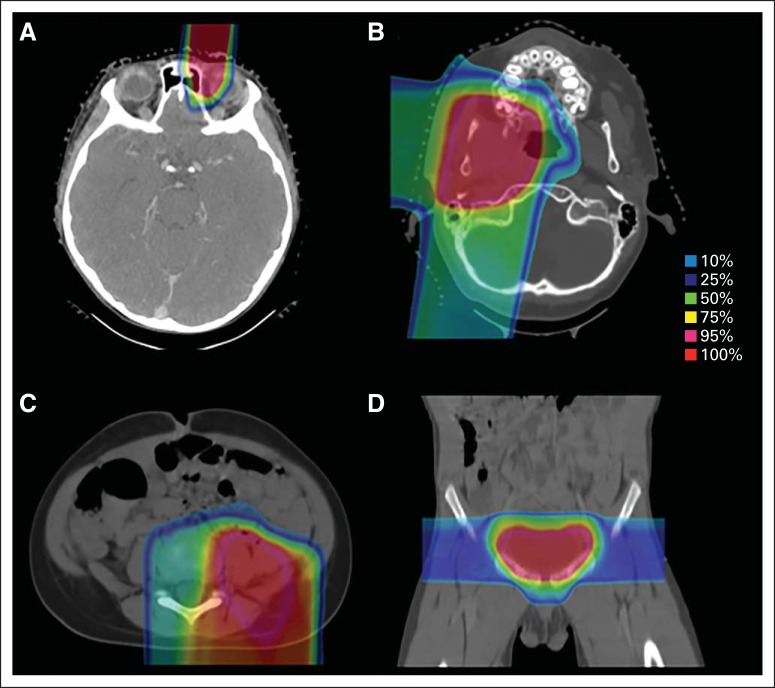
All patients received proton radiation at Massachusetts General Hospital or MD Anderson Cancer Center. Therapy was planned by using a computed tomography scan with the patient in a customized immobilization device in the treatment position. When possible, magnetic resonance imaging scans were anatomically registered to the planning computed tomography scan to better facilitate target delineation. The proton dose was prescribed in GyRBE units by using a relative biologic effectiveness of 1.1.14 Children were treated under anesthesia when necessary. Daily pretreatment image guidance was performed to ensure reproducible daily patient positioning. Site-specific examples of patient proton therapy plans are shown in Figure 1.
Fig 1.
Proton treatment plans for patients with primaries at (A) orbital, (B) parameningeal, (C) trunk, and (D) prostate sites.
Toxicity Evaluation
All patients were evaluated for acute and late effects at protocol-specified intervals. Toxicity was graded according to Common Terminology Criteria for Adverse Events, Version 3.0. In accordance with the Common Terminology Criteria for Adverse Events, acute adverse effects are toxicities occurring within 90 days of RT, and late complications are those occurring after 90 days. Acute toxicity was scored at weekly visits by the treating radiation oncologist and assessed at least once within the 3 months following completion of RT. Late toxicity was assessed annually for up to 10 years.
Statistical Analysis
The primary end points of this study were disease control and late adverse effects of treatment. Disease end points of event-free survival (EFS), overall survival (OS), and local control (LC) were measured from the date of RT start until the respective event. The failure event for EFS was the earliest date of treatment failure at local, regional, or metastatic sites or death. The treatment failure event for OS was death, and for LC, it was tumor progression at the primary site. Patients who had not experienced treatment failure with the relevant event were censored at their date of last follow-up. EFS, OS, and LC rates were estimated by using the Kaplan-Meier method with CIs constructed by using Greenwood's formula. The log-rank test was used to assess the difference in EFS, OS, and LC distributions between patients groups. Median follow-up time was calculated by the reverse Kaplan-Meier approach to the OS data. Patients were identified as low or intermediate risk according to the COG definitions at the time the protocol was written.2,3,15 Data analysis was performed by using SAS 9.3 (SAS Institute, Cary, NC), and all P values were based on a two-sided hypothesis.
RESULTS
Patient Characteristics
From February 2005 to August 2012, 57 patients were enrolled onto the study. Chemotherapy was given to 20 patients per (n = 18) or on (n = 2) the COG-D9803 (Randomized Study of Vincristine, Actinomycin-D, and Cyclophosphamide [VAC] versusVAC alternating with Vincristine, Topotecan and Cyclophosphamide for Patients with Intermediate-Risk Rhabdomyosarcoma) and COG-D9602 (Actinomycin D and Vincristine With or Without Cyclophosphamide And Radiation Therapy, for Newly Diagnosed Patients With Low-Risk Embryonal/Botryoid Rhabdomyosarcoma: An IRS-V/STS Protocol) protocols, 34 patients per (n = 16) or on (n = 18) the COG-ARST0331 (Vincristine, Dactinomycin, and Lower Doses of Cyclophosphamide With or Without Radiation Therapy for Patients with Newly Diagnosed Low-Risk Embryonal/Botryoid/Spindle Cell Rhabdomyosarcoma) and COG-ARST0531 (Randomized Study of Vincristine, Dactinomycin and Cyclophosphamide [VAC] versus VAC Alternating with Vincristine and Irinotecan [VI] for Patients with Intermediate-Risk Rhabdomyosarcoma) protocols, and three patients on the EpSSG 2005 (A Protocol for Non-Metastatic Rhabdomyosarcoma) protocol.
Patient characteristics are summarized in Table 1. Patients tended to be young, with 49% age 2 years or younger and 19% age 1 year or younger at diagnosis. Median patient age at the time of RT was 3.5 years (range, 0.6 to 19.5 years). Embryonal histology (72%) was the most common. Most tumors arose in unfavorable sites (65%), and the most common primary sites were parameningeal (PM; 47%) and orbital (23%). Intracranial extension was seen in 56% of patients with PM tumors. The median proton RT dose was 50.4 GyRBE (36 to 50.4 GyRBE).
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (N = 57) | % |
|---|---|---|
| Age, years | ||
| Median | 3.5 | |
| Range | 0.6-19.5 | |
| Male sex | 27 | 47 |
| Race/ethnicity | ||
| White (non-Hispanic) | 50 | 88 |
| All others | 7 | 12 |
| Group | ||
| I | 1 | 2 |
| II | 7 | 12 |
| III | 47 | 82 |
| IV | 2 | 4 |
| Stage | ||
| 1 | 18 | 32 |
| 2 | 14 | 25 |
| 3 | 23 | 40 |
| 4 | 2 | 3 |
| Histology | ||
| Embryonal/botryoid | 41 | 72 |
| Alveolar/undifferentiated | 16 | 28 |
| Risk group | ||
| Low | 15 | 26 |
| Intermediate | 42 | 74 |
| Site | ||
| Favorable | 19 | 33 |
| Orbital | 13 | 23 |
| Head and neck | 4 | 7 |
| Perineal | 1 | 2 |
| Biliary | 1 | 2 |
| Unfavorable | 38 | 67 |
| Parameningeal | 27 | 47 |
| Bladder/prostate | 5 | 9 |
| Extremities | 3 | 5 |
| Chest/abdomen | 2 | 4 |
| Perianal | 1 | 2 |
| Size, cm | ||
| ≤ 5 | 36 | 63 |
| > 5 | 21 | 37 |
| Nodal disease | ||
| N0 | 50 | 88 |
| N1 | 7 | 12 |
| Radiation dose GyRBE | ||
| Median | 50.4 | |
| Range | 36.0-50.4 | |
Abbreviation: RBE, relative biologic effectiveness.
Treatment Outcomes
The median follow-up of survivors was 47 months (range, 14 to 102 months) from initiation of RT. At the time of analysis, 41 patients were alive and free of disease, five patients were alive with recurrence, and 11 patients had died of disease. Of the 16 patients who recurred following treatment, eight had isolated local treatment failures, one developed concurrent local and distant disease, three had regional failures, three developed distant metastases only, and one experienced local, regional, and distant failure (Table 2). There were no CNS failures. Median time to treatment failure was 9 months from RT start (range, 3 to 48 months).
Table 2.
Details of the Patients for Whom Treatment Failed
| Patient | Age (years) | Histology | Disease Site | Tumor Size (cm) | Group | RT Dose (GyRBE) | Failure Type | Time to Failure (months) | Status at Analysis |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 6 | Emb | PM-ICE | > 5 | IIIa | 50.4 | Local | 7 | Deceased |
| 18 | 2 | Emb | PM-ICE | > 5 | IIIb | 50.4 | Local | 13 | Deceased |
| 39 | 10 | Emb | PM-ICE | > 5 | IIIa | 50.4 | Local | 17 | Alive |
| 02 | 2 | Emb | PM | ≤ 5 | IIIa | 50.4 | Local | 8 | Deceased |
| 29 | 16 | Emb | PM | > 5 | IIIa | 50.4 | Local | 12 | Deceased |
| 38 | 2 | Alv | Head and neck | ≤ 5 | IIIb | 50.4 | Local | 10 | Deceased |
| 16 | 1 | Alv | Head and neck | ≤ 5 | IIc | 41.4 | Local | 24 | Alive |
| 07 | 15 | Emb | Orbit | ≤ 5 | IIIb | 45 | Local | 6 | Alive |
| 17 | 1 | Emb | Prostate | > 5 | IIIa* | 37.8 | Regional | 7 | Deceased |
| 40 | 11 | Emb | PM | ≤ 5 | IIIa | 50.4 | Regional | 15 | Alive |
| 27 | 9 | Emb | PM-ICE | > 5 | IIIa | 50.4 | Regional | 48 | Alive |
| 41 | 8 | Emb | PM-ICE | ≤ 5 | IIIa | 50.4 | Distant | 3 | Deceased |
| 15 | 8 | Emb | PM | ≤ 5 | IIIa | 50.4 | Distant | 9 | Deceased |
| 35 | 2 | Emb | Extremity | > 5 | IIc | 41.4 | Distant | 4 | Deceased |
| 12 | 7 | Emb | PM | > 5 | IIIa | 50.4 | Local and distant | 3 | Deceased |
| 08 | 1 | Alv | Perineal | ≤ 5 | IIIa | 50.4 | Local, regional, and distant | 3 | Deceased |
Abbreviations: Alv, alveolar; Emb, embryonal; PM, parameningeal; PM-ICE, parameningeal with intracranial extension; RBE, relative biologic effectiveness; RT, radiotherapy.
Patient was Group IIIa at treatment initiation but underwent delayed primary resection.
Disease control.
Kaplan-Meier curves for EFS, OS, and LC are shown in Figure 2. For the entire cohort, the 5-year EFS was 69% and OS was 78%. The 5-year EFS and OS were 93% and 100% for low-risk patients and 61% and 70% for intermediate-risk patients, respectively (P = .04 and P = .04, respectively). Five-year EFS and OS were 92% and 100% for orbit, 60% and 69% for PM, 50% and 75% for head and neck, 80% and 80% for bladder and/or prostate, 67% and 67% for GI and/or genitourinary, and 80% and 80% for trunk and extremity sites (P = .55 and P = .54). Detailed outcomes are listed in Table 3.
Fig 2.
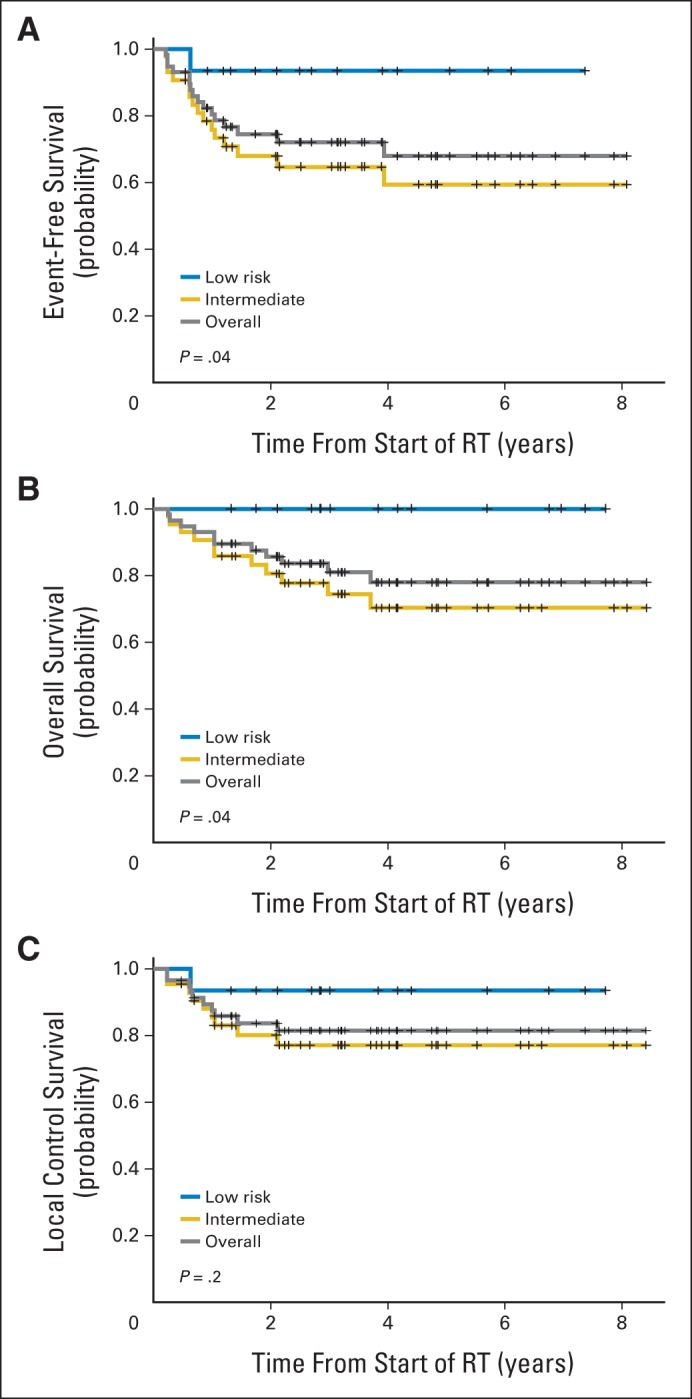
(A) Event-free, (B) overall, and (C) local control survival for the entire cohort and by risk group. RT, radiation therapy.
Table 3.
Treatment Outcomes for All Patients
| Variable | No. of Patients | 3-Year EFS |
5-Year EFS |
P | 3-Year OS |
5-Year OS |
P | 3-Year LC |
5-Year LC |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |||||
| All patients | 57 | 73 | 59 to 83 | 69 | 54 to 81 | 81 | 67 to 89 | 78 | 63 to 87 | 81 | 68 to 90 | 81 | 68 to 90 | |||
| Stage | .06 | .02 | .19 | |||||||||||||
| 1 to 2 | 32 | 81 | 62 to 91 | 81 | 62 to 91 | 93 | 76 to 98 | 88 | 66 to 96 | 87 | 69 to 95 | 87 | 69 to 95 | |||
| 3 to 4 | 25 | 63 | 41 to 79 | 55 | 31 to 74 | 64 | 40 to 81 | 64 | 40 to 81 | 73 | 49 to 87 | 73 | 49 to 87 | |||
| Group | .83 | .65 | .70 | |||||||||||||
| I to II | 8 | 73 | 28 to 93 | 73 | 28 to 93 | 88 | 39 to 98 | 88 | 39 to 98 | 83 | 27 to 97 | 83 | 27 to 97 | |||
| III to IV | 49 | 73 | 58 to 83 | 69 | 53 to 81 | 80 | 65 to 89 | 76 | 60 to 87 | 81 | 67 to 90 | 81 | 67 to 90 | |||
| Site (1) | .32 | .19 | .79 | |||||||||||||
| Favorable | 19 | 79 | 54 to 92 | 79 | 54 to 92 | 89 | 64 to 97 | 89 | 64 to 97 | 79 | 54 to 92 | 79 | 54 to 92 | |||
| Unfavorable | 38 | 70 | 52 to 82 | 64 | 45 to 79 | 76 | 58 to 88 | 71 | 51 to 84 | 83 | 65 to 92 | 83 | 65 to 92 | |||
| Site (2) | .55 | .54 | .31 | |||||||||||||
| Orbit | 13 | 92 | 57 to 99 | 92 | 57 to 99 | 100 | 100 to 100 | 100 | 100 to 100 | 92 | 57 to 99 | 92 | 57 to 99 | |||
| Parameningeal | 27 | 66 | 45 to 81 | 60 | 37 to 77 | 76 | 53 to 88 | 69 | 45 to 85 | 77 | 56 to 89 | 77 | 56 to 89 | |||
| Head and neck | 4 | 50 | 6 to 84 | 50 | 6 to 84 | 75 | 13 to 96 | 75 | 13 to 96 | 50 | 6 to 84 | 50 | 6 to 84 | |||
| Bladder and/or prostate | 5 | 80 | 20 to 97 | 80 | 20 to 97 | 80 | 20 to 97 | 80 | 20 to 97 | 100 | 100 to 100 | 100 | 100 to 100 | |||
| GI/genitourinary | 3 | 67 | 5 to 95 | 67 | 5 to 95 | 67 | 5 to 95 | 67 | 5 to 95 | 67 | 5 to 95 | 67 | 5 to 95 | |||
| Trunk/extremities | 5 | 80 | 20 to 97 | 80 | 20 to 97 | 80 | 20 to 97 | 80 | 20 to 97 | 100 | 100 to 100 | 100 | 100 to 100 | |||
| Histology | .32 | .39 | .97 | |||||||||||||
| Embryonal/botryoid | 41 | 70 | 54 to 82 | 65 | 47 to 79 | 78 | 61 to 89 | 74 | 55 to 86 | 82 | 66 to 91 | 82 | 66 to 91 | |||
| Alveolar/undifferentiated | 16 | 81 | 51 to 93 | 81 | 51 to 93 | 87 | 57 to 97 | 87 | 57 to 98 | 81 | 51 to 93 | 81 | 51 to 93 | |||
| Risk group | .04 | .04 | .20 | |||||||||||||
| Low | 15 | 93 | 61 to 99 | 93 | 61 to 99 | 100 | 100 to 100 | 100 | 100 to 100 | 93 | 61 to 99 | 93 | 61 to 99 | |||
| Intermediate | 42 | 66 | 49 to 78 | 61 | 43 to 75 | 74 | 57 to 85 | 70 | 52 to 83 | 77 | 60 to 87 | 77 | 60 to 87 | |||
| Age, years | .60 | .91 | .07 | |||||||||||||
| 2-10 | 43 | 77 | 61 to 87 | 72 | 53 to 84 | 83 | 68 to 92 | 79 | 61 to 89 | 88 | 74 to 95 | 88 | 74 to 95 | |||
| < 2 and > 10 | 14 | 64 | 34 to 83 | 64 | 34 to 83 | 76 | 42 to 92 | 76 | 42 to 92 | 64 | 34 to 83 | 64 | 34 to 83 | |||
| Size, cm | .19 | .14 | .28 | |||||||||||||
| ≤ 5 | 36 | 77 | 60 to 88 | 77 | 60 to 88 | 89 | 72 to 96 | 84 | 64 to 93 | 86 | 69 to 94 | 86 | 69 to 94 | |||
| > 5 | 21 | 65 | 41 to 82 | 56 | 29 to 76 | 66 | 38 to 84 | 66 | 38 to 84 | 74 | 47 to 88 | 74 | 47 to 88 | |||
Abbreviations: EFS, event-free survival; LC, local control; OS, overall survival.
LC.
The LC rate at 5 years was 81%. Of the 10 local (treatment) failures (LFs), six patients experienced LFs at PM, two at non-PM head and neck, one at orbital, and one at a perineal site(s) (Table 2). All LFs were within the high-dose RT region; there were no marginal treatment failures. The three isolated regional treatment failures occurred in nodal sites (two in the cervical neck and one in the inguinal region) outside the radiation portals. All six patients with PM LFs received a total dose of 50.4 GyRBE. Five of these six patients had tumors larger than 5 cm, and three patients had intracranial extension. The two patients with non-PM head and neck tumors with LFs were young (ages 1 and 2 years), had alveolar histology, and one had node-positive disease. The patient with an orbital LF received 45 GyRBE and had embryonal histology. The patient with a perineal LF had an unresected primary, alveolar histology, and was younger than age 1 year.
No variation in RT target volume coverage was observed for patients with LF compared with those with LC. The median clinical target volume (CTV) V95 (percent of CTV receiving at least 95% of the prescription dose) was 100% for both groups. A log-rank test showed no difference in LC rates for patients with CTV coverage by the prescription dose of more than 95% compared with those who had coverage of 95% or less (P = .62).
The 5-year LC rate was 93% for low-risk patients and 77% for intermediate-risk patients (P = .20). The majority of patients (83%) had group III disease, largely accounted for by the predominance of PM and orbital tumors. The 5-year LC rates were 83% for group I to II and 81% for group III to IV disease (P = .70). Actuarial LC by site was 92% for orbit, 77% for PM, 50% for non-PM head and neck, 100% for bladder and/or prostate, 67% for GI and/or genitourinary, and 100% for trunk and extremity sites (P = .31). No significant difference in LC was observed for favorable sites (v unfavorable sites) and embryonal histology (v alveolar histology). There was a trend toward improved LC for patients age 2 to 10 years compared with patients younger than 2 years or older than 10 years (88% v 64%; P = .07; Table 3).
Treatment-Related Toxicity
A total of 43 patients were included in the evaluation of late toxicity, with 14 patients excluded as a result of disease recurrence (Table 4). Ninety percent of patients had toxicity follow-up of 2 years or more and 42% had toxicity follow-up of 5 years or more.
Table 4.
Acute and Late Toxicity
| Toxicity | No. of Evaluable Patients | Grade 2 |
Grade 3 |
||
|---|---|---|---|---|---|
| No. of Observed Toxicities | Percentage of Patients With Toxicities | No. of Observed Toxicities | Percentage of Patients With Toxicities | ||
| Acute toxicity | 57 | ||||
| Orbital | 13 | ||||
| Radiation dermatitis | 5 | 38 | 1 | 8 | |
| Dry eye | 2 | 15 | 1 | 8 | |
| Head and neck | 31 | ||||
| Odynophagia | 4 | 13 | 3 | 10 | |
| Radiation dermatitis | 10 | 32 | 2 | 6 | |
| Mucositis | 19 | 61 | 1 | 3 | |
| Dry eye | 2 | 6 | 1 | 3 | |
| Otitis | 1 | 3 | 1 | 3 | |
| GI/genitourinary | 8 | ||||
| Elevated liver function tests | 0 | 0 | 1 | 13 | |
| Radiation dermatitis | 2 | 25 | 0 | 0 | |
| Diarrhea | 2 | 25 | 0 | 0 | |
| Bladder spasm | 1 | 13 | 0 | 0 | |
| Painful bowel movement | 1 | 13 | 0 | 0 | |
| Trunk/extremity | 5 | ||||
| Radiation dermatitis | 1 | 20 | 2 | 40 | |
| All Patients | 57 | ||||
| Fatigue | 3 | 5 | 0 | 0 | |
| Total | 57 | 61 | 13 | 17 | |
| Late toxicity | 43 | ||||
| Orbital | 12 | ||||
| Cataract | 0 | 0 | 1 | 8 | |
| Dry eye | 2 | 17 | 0 | 0 | |
| Facial hypoplasia/asymmetry | 1 | 8 | 0 | 0 | |
| Epistaxis | 1 | 8 | 0 | 0 | |
| Dry skin | 1 | 8 | 0 | 0 | |
| Head and neck | 21 | ||||
| Chronic otitis | 1 | 5 | 1 | 5 | |
| Retinopathy | 0 | 0 | 1 | 5 | |
| Endocrine abnormalities | 3 | 14 | 0 | 0 | |
| Cerumen buildup | 3 | 14 | 0 | 0 | |
| Facial hypoplasia/asymmetry | 2 | 10 | 0 | 0 | |
| Hearing loss (unilateral) | 2 | 10 | 0 | 0 | |
| Cavernoma | 1 | 5 | 0 | 0 | |
| Cognitive disturbance | 1 | 5 | 0 | 0 | |
| Dry eye | 1 | 5 | 0 | 0 | |
| Trunk/extremity | 4 | ||||
| Skeletal or muscle defect | 1 | 25 | 0 | 0 | |
| Total | 20 | 28 | 3 | 7 | |
NOTE. Fifty-seven acute grade 2 toxicities developed in 35 patients, 13 acute grade 3 toxicities developed in 11 patients, 20 late grade 2 toxicities developed in 12 patients, and 3 late grade 3 toxicities developed in three patients.
No patient died as a result of RT-related toxicity, and there were no acute or late toxicities greater than grade 3 (Table 4). Eleven patients (13%) experienced acute grade 3 toxicities attributable to RT, the most common of which were odynophagia (10% for patients with PM and/or head and neck sites) and RT dermatitis (9% for all patients). Grade 3 mucositis was seen in one patient. All RT-related mucositis and dermatitis resolved completely after treatment without infectious complications or prolonged weight loss. Three patients (7%) developed late grade 3 toxicity consisting of a unilateral cataract (orbital primary), chronic otitis (PM mastoid primary), and retinopathy with decreased visual acuity (orbital primary).
There were 20 incidents of late grade 2 toxicity in 12 patients (28%). Endocrine dysfunction related to RT dose to the hypothalamus or pituitary was seen in three patients with PM sites (growth hormone deficiency in two patients, combined thyroid and growth hormone deficiency in one patient). Three patients developed mild grade 2 facial hypoplasia, two were treated for PM tumors at ages 3 and 4 years, and one with an orbital tumor was treated at age 7 years. Three patients developed grade 2 dry eye defined as symptomatic requiring intervention (eg, lubricating drops) without decreased visual acuity. Grade 2 unilateral hearing loss (defined as hearing loss not requiring hearing aid or intervention) was seen in two patients with PM sites. One patient treated at age 2 years for a PM tumor with extensive intracranial extension (ICE) developed grade 2 effects on memory and processing speed 8 years after treatment. One patient with a lower extremity tumor developed muscular atrophy (grade 2) after surgery and RT. To date, there have been no reported secondary malignancies.
DISCUSSION
This phase II study represents, to the best of our knowledge, the first reported prospective trial investigating the use of protons for pediatric RMS. It was undertaken to evaluate the efficacy of proton therapy for disease control and to assess acute and late adverse effects in the setting of potentially reduced radiation dose to tissues outside the target volume.11–13
The disease control in this heterogeneous population remained comparable to published outcomes for pediatric RMS. Low-risk patients in our study had excellent outcomes with a 5-year EFS and OS of 93% and 100%, respectively, that are similar to the results published in the COG-D9602 trial, in which 5-year failure-free survival was 85% to 89%, and 5-year OS was 93% to 97%.4
For intermediate-risk patients, the 5-year EFS of 61% and OS of 70% appear lower than the 4-year failure-free survival of 68% to 73% and OS of 79% seen in the COG-D9803 trial; however, the CIs fully include the COG-D9803 outcomes.2 The majority of our intermediate-risk patients (64%) had PM primaries—a much higher incidence than the 35% PM primaries seen in the COG-D9803 trial. These tumors historically have a poorer prognosis compared with other RMS sites, with a 5-year EFS of 65% and OS of 70%.4 Because we practice at a referral center for proton therapy, we tend to treat a higher proportion of young children (19% younger than age 2 years at diagnosis) and advanced PM tumors, which may contribute to our lower survival rates. Age younger than 2 years or older than 10 years and primary tumor size more than 5 cm showed a nonsignificant trend toward decreased survival in our cohort, and tumors larger than 5 cm accounted for five of the six PM LFs. Still, our PM LF rate of 23% is comparable to that seen in Intergroup Rhabdomyosarcoma Study Group protocol IV (IRSG-IV; Intergroup Rhabdomyosarcoma Study-IV: Results for Patients With Nonmetastatic Disease) and COG-D9803, in which LF was 19% for all patients with PM tumors (15% to 28%, depending on the extent of ICE or cranial base and/or nerve involvement).16
Other recent retrospective studies looking at outcomes for PM tumors alone have reported disease control rates similar to those in our study. For PM tumors, our 3-year and 5-year EFS rates were 66% and 60% and LF rates were 23% and 23%. Of the published studies that used IMRT for LC of PM tumors, Yang et al17 reported a 5-year EFS of 58% to 61% and LF of 14%, Eaton et al18 reported a 3-year disease-free survival of 69% and LF of 8%, and Curtis et al19 found a 4-year OS of 43% and LF of 16%. Notably, these studies had a significant proportion of isolated treatment failures in the CNS, ranging from 12% to 28%,17–19 whereas our cohort had none despite 56% presenting with ICE. For patients with non-PM head and neck disease, our EFS and LC of 50% and 50% is below the 76% and 81% reported in IRSG-III and -IV, although small numbers (n = 4) and a predominance of young patients with alveolar histology likely contributed to our lower control rates.20
Site-specific outcomes for other disease sites are comparable or favorable to published results. Orbital tumors had a 5-year EFS and LC of 92% and 92%, similar to the EFS and LC of 86% to 98% from IRSG-IV and COG-D9602.3,5,21 For patients with bladder and/or prostate disease, EFS and LC were 80% and 100% in our cohort and 77% and 86% in the IRSG-IV study.22 Other GI and genitourinary (n = 3; biliary, perineal, and perianal) sites had an EFS and LC of 67% and 67%, with one patient who had alveolar perineal disease developing rapid local, regional, and distant progression after treatment. Published outcomes for pediatric biliary RMS are limited but perineal and perianal rates are reported as a 5-year EFS of 45% from IRSG-IV and a 5-year EFS and LC of 33% and 83% from the recent publication by Casey et al23 from Memorial Sloan-Kettering.24 Trunk and extremity sites in our series had an EFS and LC of 80% and 100% comparable to the 65% and 93% seen in IRSG-IV.21,25
In addition to disease control, a primary aim of our study was to describe the acute and late toxicities associated with proton RT. Grade 3 acute effects occurred at an incidence of 17% in our patient population with RT dermatitis (9% of all patients) and odynophagia (10% of patients with head and neck and PM sites) being the most common. There was only one report of acute grade 3 mucositis (6% of patients with head and neck and PM sites). When compared with the conventionally fractionated arm of IRSG-IV in which 46% of patients developed acute grade 3 to 4 mucositis and 16% developed grade 3 to 4 skin reaction, our results appear favorable.26 More recent data on acute photon toxicity with IMRT is not well described in the literature. A single retrospective German cohort of 17 pediatric patients with orbital and head and neck RMS treated with fractionated stereotactic radiotherapy and IMRT reported grade 2 erythema at 42% and grade 2 mucositis at 57%, with no grade 3 acute toxicity seen.27
Significant late toxicity in our study was minimal, with only three grade 3 late adverse effects to date. Comparisons to late toxicity rates from cohorts treated with photon radiation therapy are problematic, because the largest reports come from IRSG-II and -III in which IMRT was not used.28,29 Long-term toxicity data with IMRT is sparse and, to the best of our knowledge, is described in only three single-institution series that were limited to patients with head and neck, orbital, and PM sites. From these studies, late toxicity was seen in 32% to 47% of patients and reported as endocrine abnormalities (4% to 11%), facial hypoplasia (4% to 10%), dry eye (0% to 16%), cataracts (0% to 11%), and secondary malignancies (0% to 5%).19,27,30 In our cohort, late toxicity of any grade was seen in 15 patients (35%) and for patients with combined orbital and head and neck sites, we saw endocrine abnormalities in 9% (0% orbital and 14% head and neck and PM sites), facial hypoplasia in 9% (8% orbital and 10% head and neck and PM sites), dry eye in 9% (17% orbital and 5% head and neck and PM sites), cataracts in 3% (8% orbital and 0% head and neck and PM sites), and no secondary malignancies (Table 4). The patients in IMRT studies are not directly comparable to our cohort because of retrospective data collection, the absence of toxicity grading, inclusion of both adult and pediatric patients, and limited follow-up (only one study had more than 2 years median follow-up). Results from the ongoing COG-ARST0331 and COG-ARST0531 trials may provide the most valuable data for comparing modern photon toxicity rates with this proton-treated cohort.
To date, no secondary malignancies have been reported in our trial patients. Clinical data from mixed pediatric and adult populations of more than 1,000 patients have suggested a reduction in second tumor rates in a proton-treated population.31 However, both a larger cohort and longer follow-up will be needed to determine whether there is a reduction in the second malignancy rate of our pediatric patients with RMS treated with protons.
In conclusion, proton RT appears to be a feasible, safe, and effective modality for use in the pediatric RMS population. Early results of this prospective trial demonstrate comparable disease outcomes to those in photon-treated populations and, thus far, limited treatment-related adverse effects. Additional follow-up and prospectively collected photon toxicity data are needed to determine whether proton RT truly reduces the incidence and severity of late effects in comparison to patients treated with modern photon techniques.
Supplementary Material
Acknowledgment
The authors thank their patients for their participation in the study and also their referring teams for their continued support and cooperation. In particular, the authors thank Betty Cruz, Vernita Larue, Kat Steacy, Heidi Gosling, and Eva Widing for their efforts and Lauren Thornton and Elizabeth Weyman who were not officially part of this study but were integral to the collection of patient follow-up information.
Glossary Terms
- intensity-modulated radiation therapy:
radiation treatment using beams with nonuniform fluence profiles that shape the dose distribution in the target volume and adjacent normal structures. Beam modulation is typically achieved via multileaf collimators or custom-milled compensators to achieve the appropriate fluence profiles calculated by inverse optimization algorithms. The radiation beam is divided into beamlets of varying intensity such that the sum from multiple beams via inverse planning results in improved tumor targeting and normal tissue sparing. A technique of radiation therapy delivery in which the intensity of each beamlet of radiation coming from a specific angle can be adjusted to provide a desired dose distribution when the doses delivered from all beamlets are added from a single angle and from all dose delivery angles. An advanced type of high-precision radiotherapy, which aims to improve the coverage of the radiotherapy target and/or minimize radiation dose to surrounding normal tissue.
Footnotes
Listen to the podcast by Dr Wolden at www.jco.org/podcasts
Supported by Children's Oncology Group Chair Grant No. U10CA98543 and other philantrhopic sources (cog-foundation.org). The Children's Oncology Group is primarily funded by the National Cancer Institute and also receives additional funding from other granting agencies.
Presented in part at the 55th Annual Meeting of the American Society for Radiation Oncology, Atlanta, GA, September 22-25, 2013, and at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Nancy J. Tarbell, ProCure (U) Stock Ownership: Nancy J. Tarbell, ProCure Honoraria: None Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Beow Yong Yeap, Nancy J. Tarbell, Torunn I. Yock
Financial support: Torunn I. Yock
Administrative support: Claire P. Goebel, Torunn I. Yock
Provision of study materials or patients: Anita Mahajan, Alison M. Friedmann, Shannon M. MacDonald, David R. Grosshans, Carlos Rodriguez-Galindo, Karen J. Marcus, Nancy J. Tarbell, Torunn I. Yock
Collection and assembly of data: Matthew M. Ladra, Anita Mahajan, Alison M. Friedmann, Claire P. Goebel, Shannon M. MacDonald, David R. Grosshans, Carlos Rodriguez-Galindo, Karen J. Marcus, Nancy J. Tarbell, Torunn I. Yock
Data analysis and interpretation: Matthew M. Ladra, Jackie D. Szymonifka, Beow Yong Yeap, Torunn I. Yock
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ries LAG, Smith MA, Gurney JG, et al. United States SEER program 1975-1995, National Cancer Institute, SEER Program. NIH Pub. No. 99-4649. Bethesda: MD; 1999. Cancer Incidence and Survival Among Children and Adolescents. [Google Scholar]
- 2.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29:1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merks JH, De Salvo GL, Bergeron C, et al. Parameningeal rhabdomyosarcoma in pediatric age: Results of a pooled analysis from North American and European cooperative groups. Ann Oncol. 2014;25:231–236. doi: 10.1093/annonc/mdt426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: A report from the Children's Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83:720–726. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minn AY, Lyden ER, Anderson JR, et al. Early treatment failure in intermediate-risk rhabdomyosarcoma: Results from IRS-IV and D9803—A report from the Children's Oncology Group. J Clin Oncol. 2010;28:4228–4232. doi: 10.1200/JCO.2010.29.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 8.Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 9.Cotter SE, McBride SM, Yock TI. Proton radiotherapy for solid tumors of childhood. Technol Cancer Res Treat. 2012;11:267–278. doi: 10.7785/tcrt.2012.500295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozak KR, Adams J, Krejcarek SJ, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 11.Yock T, Schneider R, Friedmann A, et al. Proton radiotherapy for orbital rhabdomyosarcoma: Clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63:1161–1168. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 12.Childs SK, Kozak KR, Friedmann AM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: Clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82:635–642. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter SE, Herrup DA, Friedmann A, et al. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: Clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1367–1373. doi: 10.1016/j.ijrobp.2010.07.1989. [DOI] [PubMed] [Google Scholar]
- 14.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 15.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 16.Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: An analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys. 2013;87:512–516. doi: 10.1016/j.ijrobp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JC, Wexler LH, Meyers PA, et al. Parameningeal rhabdomyosarcoma: Outcomes and opportunities. Int J Radiat Oncol Biol Phys. 2013;85:e61–e66. doi: 10.1016/j.ijrobp.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Eaton BR, McDonald MW, Kim S, et al. Radiation therapy target volume reduction in pediatric rhabdomyosarcoma: Implications for patterns of disease recurrence and overall survival. Cancer. 2013;119:1578–1585. doi: 10.1002/cncr.27934. [DOI] [PubMed] [Google Scholar]
- 19.Curtis AE, Okcu MF, Chintagumpala M, et al. Local control after intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2009;73:173–177. doi: 10.1016/j.ijrobp.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Pappo AS, Meza JL, Donaldson SS, et al. Treatment of localized nonorbital, nonparameningeal head and neck rhabdomyosarcoma: Lessons learned from Intergroup Rhabdomyosarcoma Studies III and IV. J Clin Oncol. 2003;21:638–645. doi: 10.1200/JCO.2003.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 22.Arndt C, Rodeberg D, Breitfeld PP, et al. Does bladder preservation (as a surgical principle) lead to retaining bladder function in bladder/prostate rhabdomyosarcoma? Results from Intergroup Rhabdomyosarcoma Study IV. J Urol. 2004;171:2396–2403. doi: 10.1097/01.ju.0000127752.41749.a4. [DOI] [PubMed] [Google Scholar]
- 23.Casey DL, Wexler LH, LaQuaglia MP, et al. Patterns of failure for rhabdomyosarcoma of the perineal and perianal region. Int J Radiat Oncol Biol Phys. 2014;89:82–87. doi: 10.1016/j.ijrobp.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 24.Blakely ML, Andrassy RJ, Raney RB, et al. Prognostic factors and surgical treatment guidelines for children with rhabdomyosarcoma of the perineum or anus: A report of Intergroup Rhabdomyosarcoma Studies I through IV, 1972 through 1997. J Pediatr Surg. 2003;38:347–353. doi: 10.1053/jpsu.2003.50106. [DOI] [PubMed] [Google Scholar]
- 25.Neville HL, Andrassy RJ, Lobe TE, et al. Preoperative staging, prognostic factors, and outcome for extremity rhabdomyosarcoma: A preliminary report from the Intergroup Rhabdomyosarcoma Study IV (1991-1997) J Pediatr Surg. 2000;35:317–321. doi: 10.1016/s0022-3468(00)90031-9. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—A report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 27.Combs SE, Behnisch W, Kulozik AE, et al. Intensity Modulated Radiotherapy (IMRT) and Fractionated Stereotactic Radiotherapy (FSRT) for children with head-and-neck-rhabdomyosarcoma. BMC Cancer. 2007;7:177. doi: 10.1186/1471-2407-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and -III—IRS Group of the Children's Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33:362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Raney RB, Anderson JR, Kollath J, et al. Late effects of therapy in 94 patients with localized rhabdomyosarcoma of the orbit: Report from the Intergroup Rhabdomyosarcoma Study (IRS)-III, 1984-1991. Med Pediatr Oncol. 2000;34:413–420. doi: 10.1002/(sici)1096-911x(200006)34:6<413::aid-mpo6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Wolden SL, Wexler LH, Kraus DH, et al. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Chung CS, Yock TI, Nelson K, et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.