Figure 1.
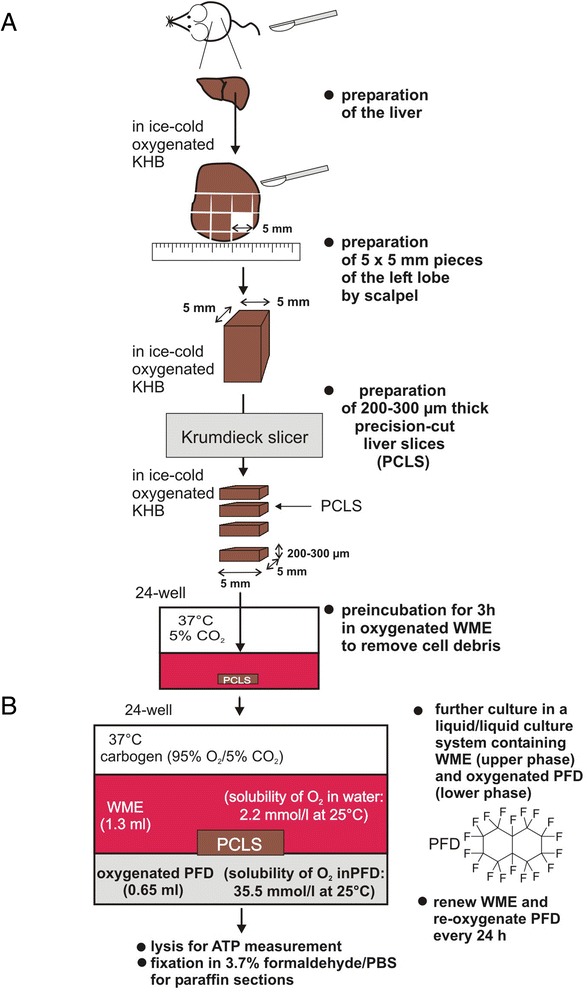
Workflow for generation of murine PCLS. (A): Preparation of murine precision-cut liver slices. Mouse livers were transferred into oxygenated ice-cold KHB. The left liver lobe was separated and placed into a petri dish containing ice-cold KHB. The lobe was cut into pieces of approximately 5 × 5 mm using a surgical blade. These pieces were further cut into PCLS of 200–300 μm thickness using a Krumdieck tissue slicer filled with ice-cold KHB using medium arm speed and blade speed. (B): PFD containing liquid/liquid culture system. After washing, PCLS were transferred into a liquid/liquid culture system. This culture system consists of WME (upper phase) and non-water soluble PFD (lower phase, density: 1.908 g/ml at 25°C) that was oxygenated using carbogen (95% O2 and 5% CO2). PCLS float on the PFD phase that provides oxygen from the bottom. WME was exchanged, and PFD was re-oxygenated every 24 h. PCLS were cultured up to 3 days in carbogen atmosphere at 37°C gently shaking the culture plates.
