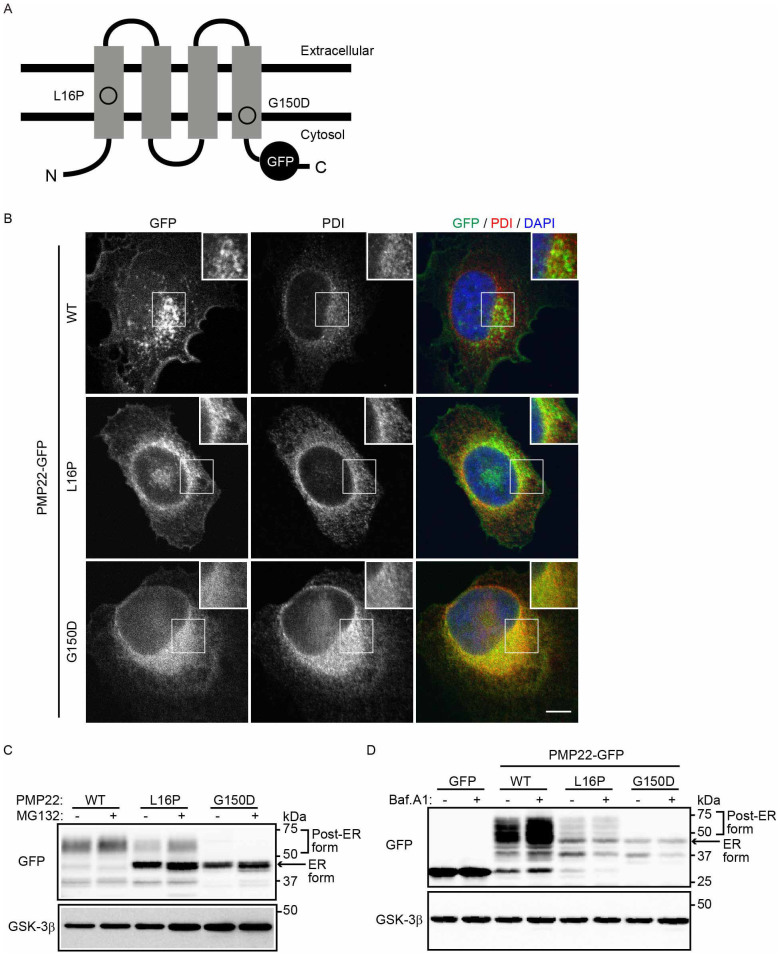
Figure 1. Disease-associated PMP22 mutants are retained in the ER and degraded in part by the proteasome-dependent degradation pathway.
(A) Structure of PMP22-GFP. The positions of each mutation in PMP22 are illustrated. (B) Localization of PMP22-GFP in HeLa cells. HeLa cells stably expressing wild-type (WT) or mutant PMP22-GFP (green) were immunostained using an anti-PDI antibody (red) and DAPI (blue) and observed using confocal laser scanning microscopy. Scale bar, 10 μm. (C) The effect of a proteasome inhibitor on the stability of PMP22. HeLa cells stably expressing WT or mutant PMP22-GFP were cultured for 8 h in the presence or absence of 10 μM MG132. The cell lysates were immunoblotted with the indicated antibodies. (D) Lysosomal degradation of WT PMP22. HeLa cells stably expressing WT or mutant PMP22-GFP were cultured for 16 h in the presence or absence of 100 nM bafilomycin A1 (Baf. A1). The immunoblots of cell lysates were probed with the indicated antibodies. Note that cropped western blots are shown, and full-length images are presented in the supplementary information.