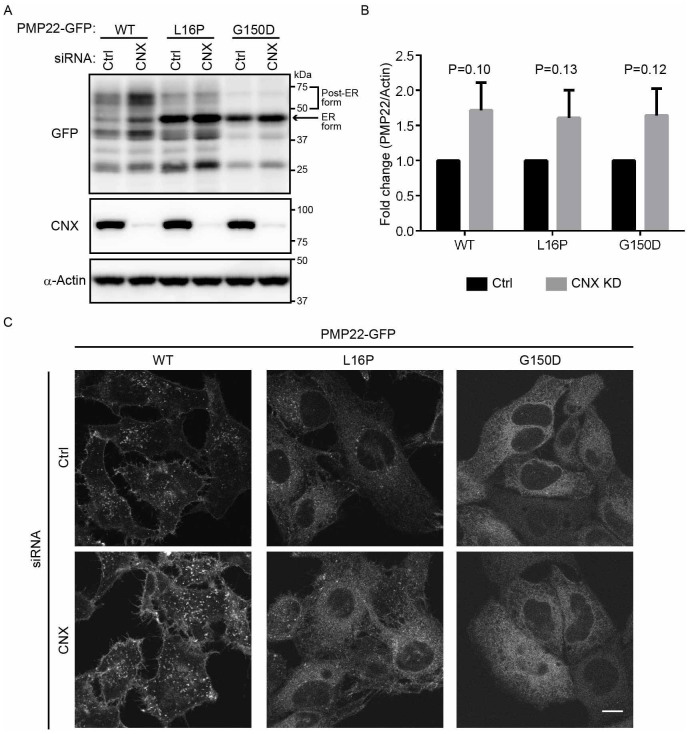
Figure 5. Mutant PMP22 is primarily retained in the ER in calnexin-knockdown cells.
(A) The effect of calnexin knockdown on the stability of ER retention of WT and mutant PMP22-GFPs. HeLa cells stably expressing WT or mutant PMP22-GFP were transfected with control or siRNAs against calnexin for 3 days. Lysates prepared from these cells were immunoblotted using anti-GFP (top panel), anti-calnexin (CNX, middle panel), and anti-α-actin (bottom panel) antibodies. Note that cropped western blots are shown, and full-length images are presented in the supplementary information. (B) The signal intensities of each PMP22-GFP derivative and α-actin band were quantified using image J software, and the amount of each PMP22-GFP derivative was normalised to the amount of α-actin. To compare the amounts of PMP22-GFP derivatives in control cells with that in calnexin-knockdown cells, we calculated the fold changes by expressing each normalised value relative to the normalised value obtained with each PMP22-GFP derivative in control cells. Values indicate the mean ± SEM of three independent experiments. Student's t-test was used to determine the significance of the differences. (C) The effect of calnexin knockdown on the localization of PMP22-GFP derivatives. HeLa cells stably expressing WT or mutant PMP22-GFP were transfected with control or siRNAs against calnexin and cultured for 3 days. Then, cells were fixed and observed using confocal laser scanning microscopy. Scale bar, 10 μm.