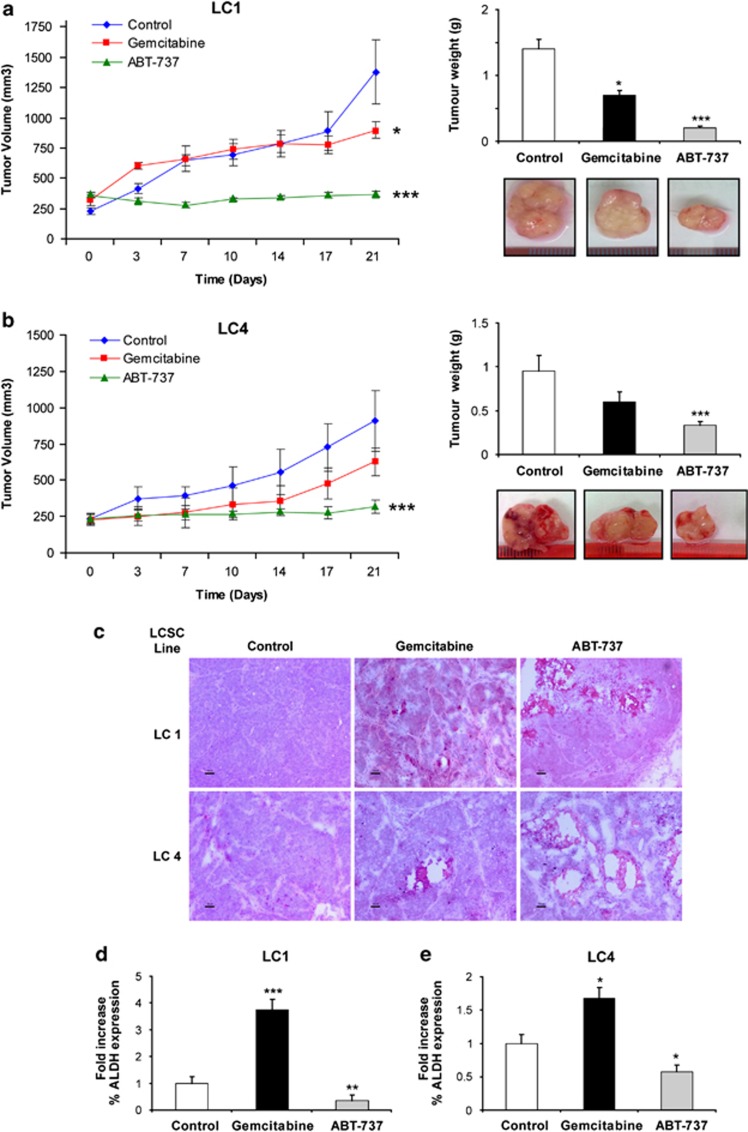
Figure 6.
ABT-737 blocks the growth of LCSC-derived xenografts and reduces LCSC content in vivo. (a) Growth of tumor xenografts derived from the LCSC line LC1 in mice treated with vehicle (Control), gemcitabine or ABT-737 as described in Materials and Methods (left), tumor weight at the end of the experiment and representative picture of the tumors (right). Bars represent mean±S.D.; *P≤0.05 and ***P≤0.001 (n=3). (b) Growth of tumor xenografts derived from the LCSC line LC4 and treated as above (left), tumor weight at the end of the experiment and representative picture of the tumors (right). Bars represent mean±S.D.; ***P≤0.001 (n=3). (c) Hematoxylin–eosin-stained sections of xenografts derived from the LCSC lines LC1 and LC4 after 3 weeks of treatment with vehicle (Control), gemcitabine or ABT-737 as described above; × 10 magnification, bar 50 μm. (d) Fold variation of ALDEFLUOR-positive cells in gemcitabine- and ABT-737-treated tumor xenografts obtained with the LC1 LCSC line as compared with vehicle-treated controls. Bars represent mean±S.D.; **P≤0.01 and ***P≤0.001 (n=3). (e) Fold variation of ALDEFLUOR-positive cells in gemcitabine and ABT-737-treated tumor xenografts obtained with the LC4 LCSC line as compared with vehicle-treated controls. Bars represent mean±S.D.; *P≤0.05