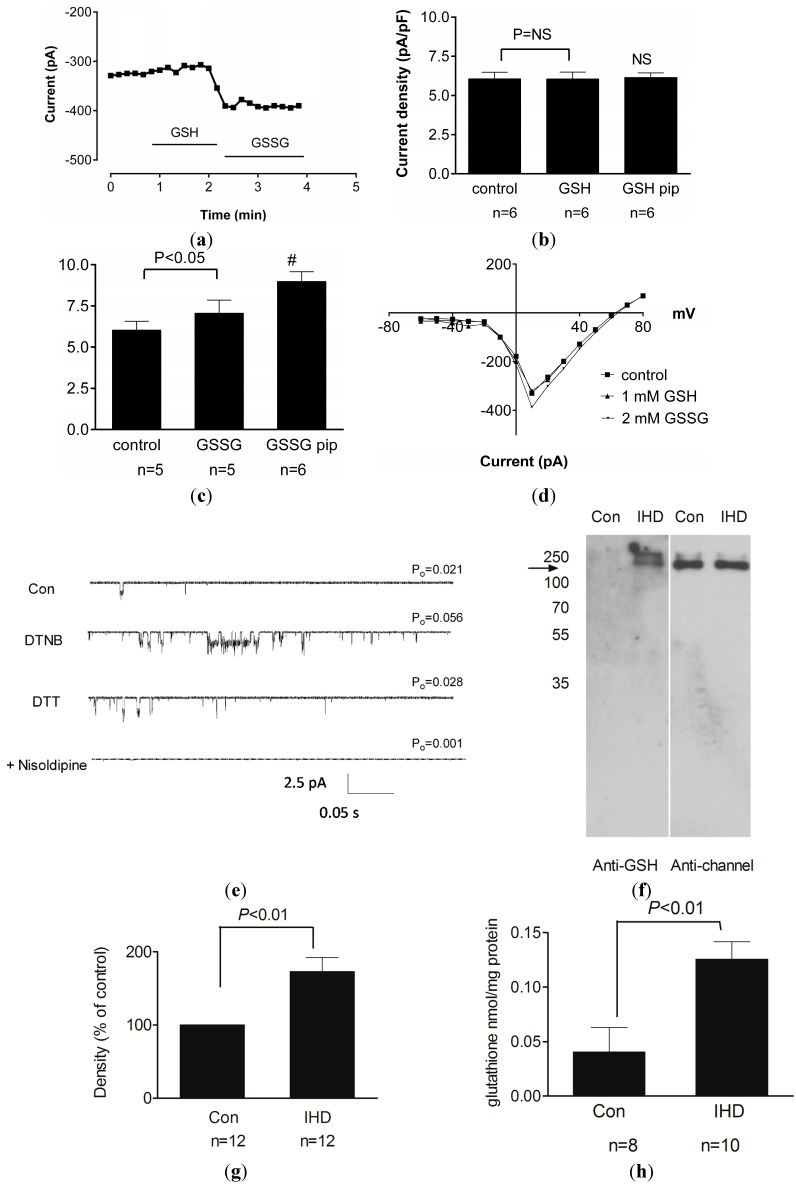
Figure 3.
Oxidative stress causes persistent enhancement of the L-type Ca2+ channel via ROS-mediated glutathionylation. (a) Time course of changes in membrane current recorded from guinea pig cardiac myocytes during extracellular exposure to 1 mM GSH followed by 2 mM GSSG as indicated; (b) Mean (±SEM) of L-type Ca2+ channel current density under control conditions (no drugs) and after exposure to GSH applied either extracellularly or in the patch pipette (GSH pip). NS, not significant; (c) Mean (±SEM) of L-type Ca2+ channel current density under control conditions (no drugs) and after exposure to GSSG applied either extracellularly or in the patch pipette (GSSG pip). #, p < 0.05 vs. control; (d) Current-voltage relationship for representative myocytes during voltage steps from −60 to +80 mV and exposure to GSH or GSSG as indicated; (e) Representative single channel currents recorded at −100 mV in the absence and presence of 200 µM DTNB followed by 1 mM DTT and then 2 µM nisoldipine. The open probability (P0) for each treatment is indicated; (f) Immunoblot demonstrating glutathionylation of protein after probing with anti-GSH antibody (left) and anti-channel antibody (right) in immunoprecipitated Cav1.2 channel protein samples from control nonischemic human heart (Con) and ischemic human heart (IHD); (g) Densitometry analysis of immunoblots for glutathionylated protein band normalised to the channel protein in the same lane for Con samples and IHD samples (mean ± SEM); and (h) Concentration of protein-glutathione mixed-disulfides in channel protein from Con and IHD heart samples (mean ± SEM). Reproduced with permission from [74].