Figure 5.
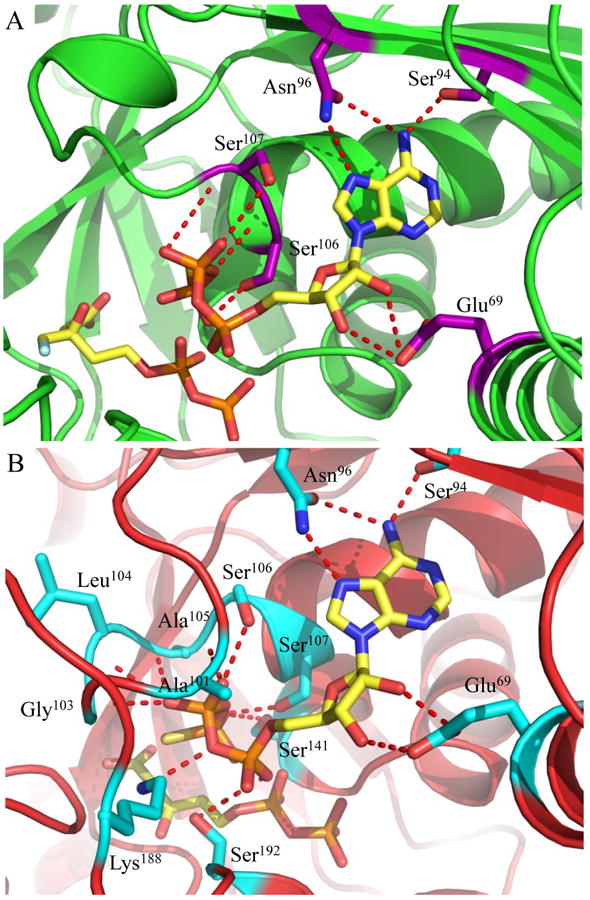
Active sites of ternary co-crystal structures from mutant forms of MDD. (A) 1.90 Å co-crystal structure of S. epidermidis S192A MDD in cartoon format (green). Inhibitor FMVAPP and nucleotide analog ATPγS are represented as ball and stick (yellow). Active site side chains within interaction distance of ATPγS are depicted in ball and stick (purple). (B) 2.60 Å co-crystal structure of S. epidermidis D283A MDD in cartoon format (red). Substrate MVAPP and nucleotide analog ATPγS are represented as ball and stick (yellow). Active site side chains within interaction distance of ATPγS are depicted in ball and stick (cyan). Hydrogen bonding distances can be found in Supplemental Table 3.
